Catalyst for alkylation separation of m-cresol and p-cresol and separation method
A catalyst, p-cresol technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problem that catalysts cannot be recycled well, cannot be well Control side reactions, increase production costs, etc., to achieve the effects of low price, easy separation, and low equipment corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
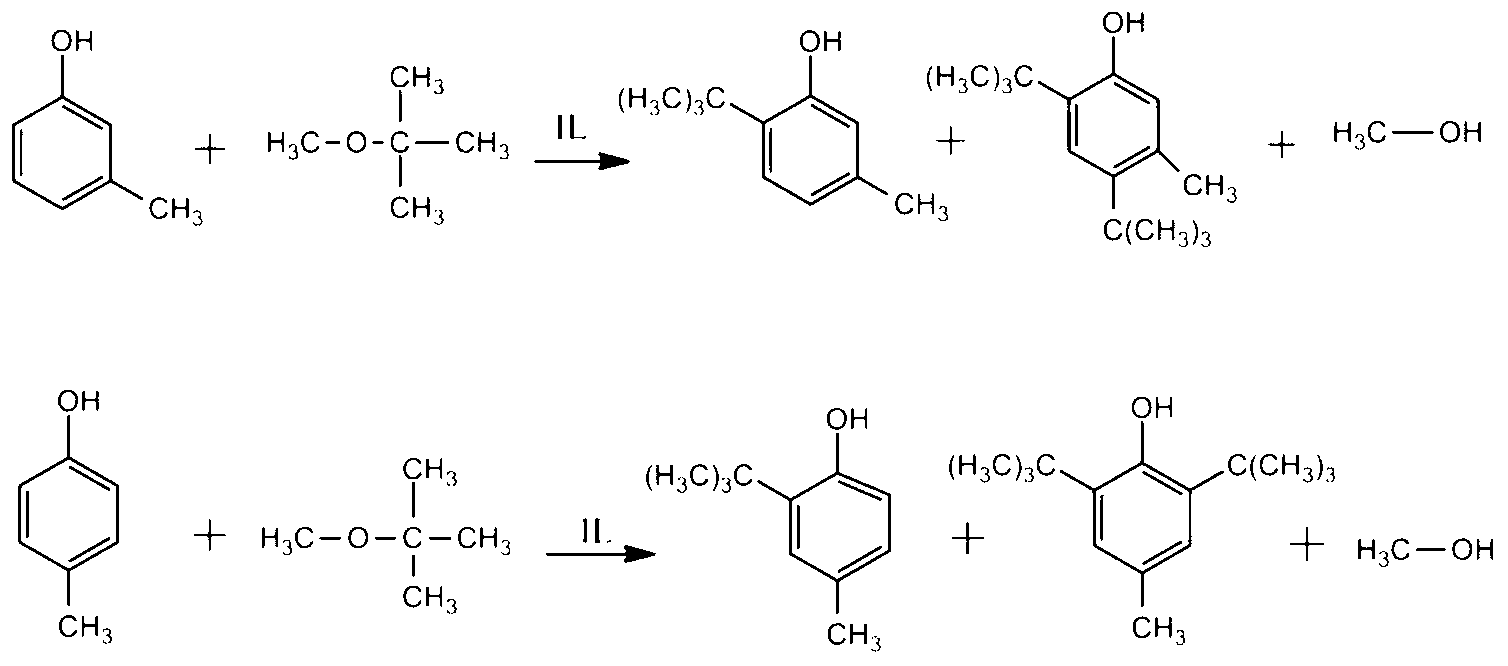
[0027] m-p-cresol mixture (m-cresol content 50%), the reaction ratio is cresol: 4-(3-methyl-1-imidazole)-1-butylsulfonic acid bisulfate: MTBE=1:0.2: 4 (molar ratio), reaction temperature 80 ℃, reaction time 8h. The reaction product contains 6-tert-butyl-m-cresol, 2-tert-butyl-p-cresol, 4,6-di-tert-butyl-m-cresol and 2,6-di-tert-butyl-p-cresol. The conversion rates of m-cresol and p-cresol were 89.00% and 79.05%, respectively, and the selectivities of 4,6-di-tert-butyl-m-cresol and 2,6-di-tert-butyl-p-cresol were 26.92% and 18.05%, respectively. %. No cresol hydroxyl hydrogen substitution products (tert-butyl m-cresol ether and tert-butyl p-cresol ether) were produced in the reaction product. Add 3.5 times the volume of cyclohexane to the reaction product, shake it up and let it stand for 4 hours, the catalyst layer and the cyclohexane layer containing the hydrocarbonated product can be clearly separated, indicating that the catalyst is easy to separate from the product and r...
example 2
[0030] m-p-cresol mixture (m-cresol content 50%), the reaction ratio is cresol: 4-(3-methyl-1-imidazole)-1-butylsulfonic acid bisulfate: MTBE=1:0.1: 3 (molar ratio), reaction temperature 75 ℃, reaction time 7h. The reaction product contains 6-tert-butyl-m-cresol, 2-tert-butyl-p-cresol, 4,6-di-tert-butyl-m-cresol and 2,6-di-tert-butyl-p-cresol. The conversion rates of m-cresol and p-cresol were 84.56% and 78.05%, respectively, and the selectivities of 4,6-di-tert-butyl-m-cresol and 2,6-di-tert-butyl-p-cresol were 24.19% and 17.02%, respectively. %. No cresol hydroxyl hydrogen substitution products (tert-butyl m-cresol ether and tert-butyl p-cresol ether) were produced in the reaction product. Add 3.5 times the volume of cyclohexane to the reaction product, shake it up and let it stand for 4 hours, the catalyst layer and the cyclohexane layer containing the hydrocarbonated product can be clearly separated, indicating that the catalyst is easy to separate from the product and r...
example 3
[0032]m-p-cresol mixture (m-cresol content 50%), the reaction ratio is cresol: 4-(3-methyl-1-imidazole)-1-butylsulfonic acid bisulfate: MTBE=1:0.3: 3 (molar ratio), reaction temperature 90 ℃, reaction time 9h. The reaction product contains 6-tert-butyl-m-cresol, 2-tert-butyl-p-cresol, 4,6-di-tert-butyl-m-cresol and 2,6-di-tert-butyl-p-cresol. The conversion rates of m-cresol and p-cresol were 88.90% and 79.45%, respectively, and the selectivities of 4,6-di-tert-butyl-m-cresol and 2,6-di-tert-butyl-p-cresol were 26.93% and 18.67%, respectively. %. No cresol hydroxyl hydrogen substitution products (tert-butyl m-cresol ether and tert-butyl p-cresol ether) were produced in the reaction product. Add 3.5 times the volume of cyclohexane to the reaction product, shake it up and let it stand for 4 hours, the catalyst layer and the cyclohexane layer containing the hydrocarbonated product can be clearly separated, indicating that the catalyst is easy to separate from the product and re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com