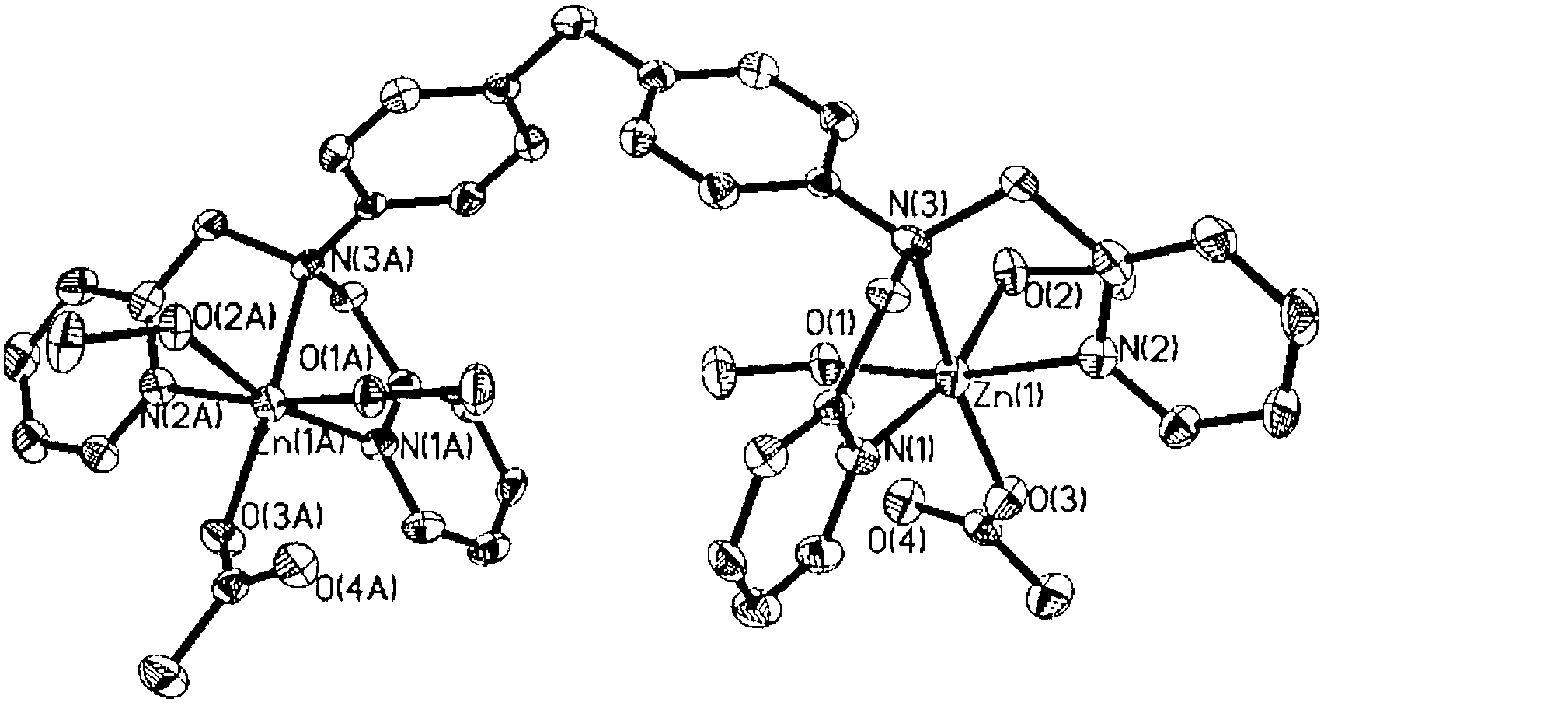
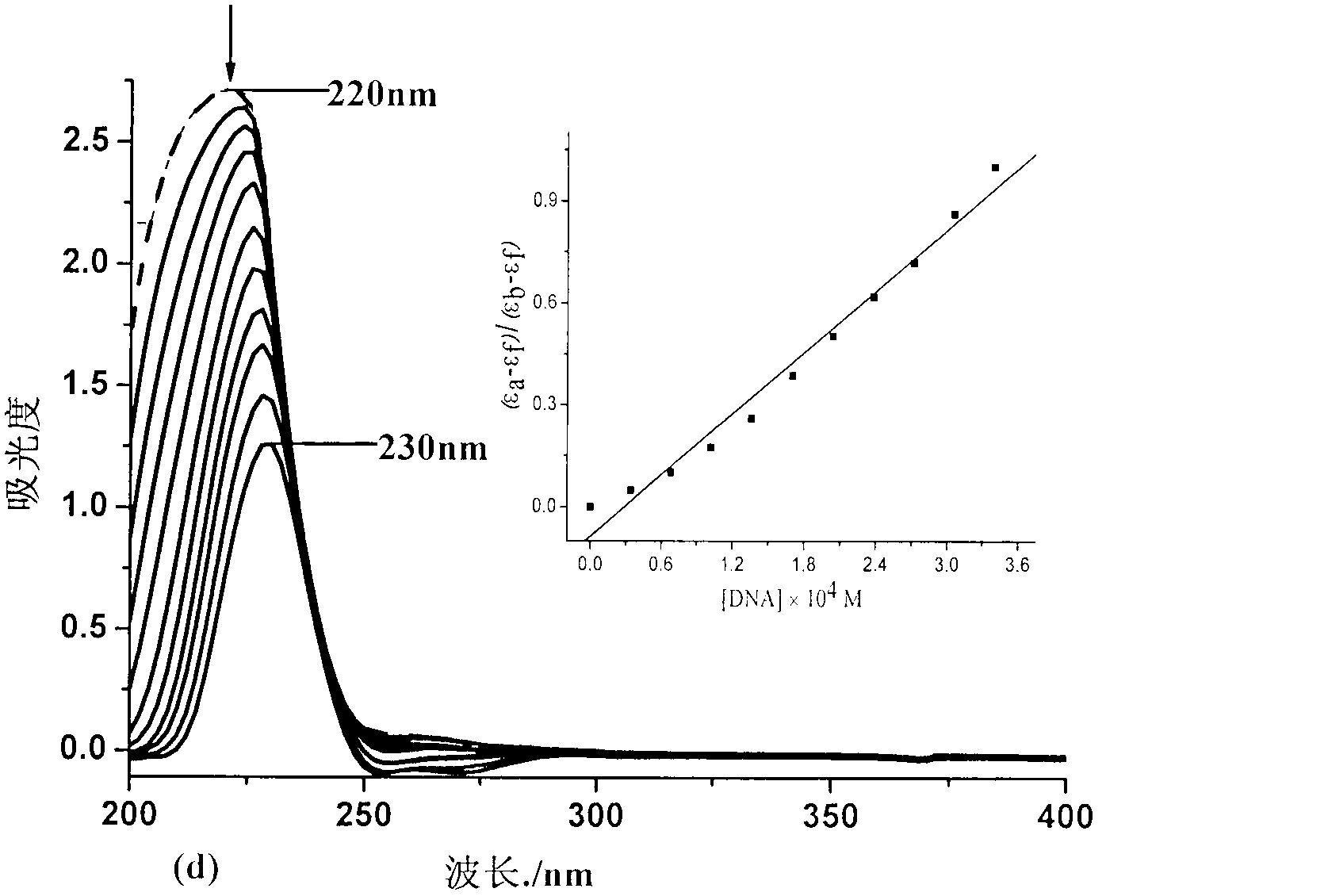
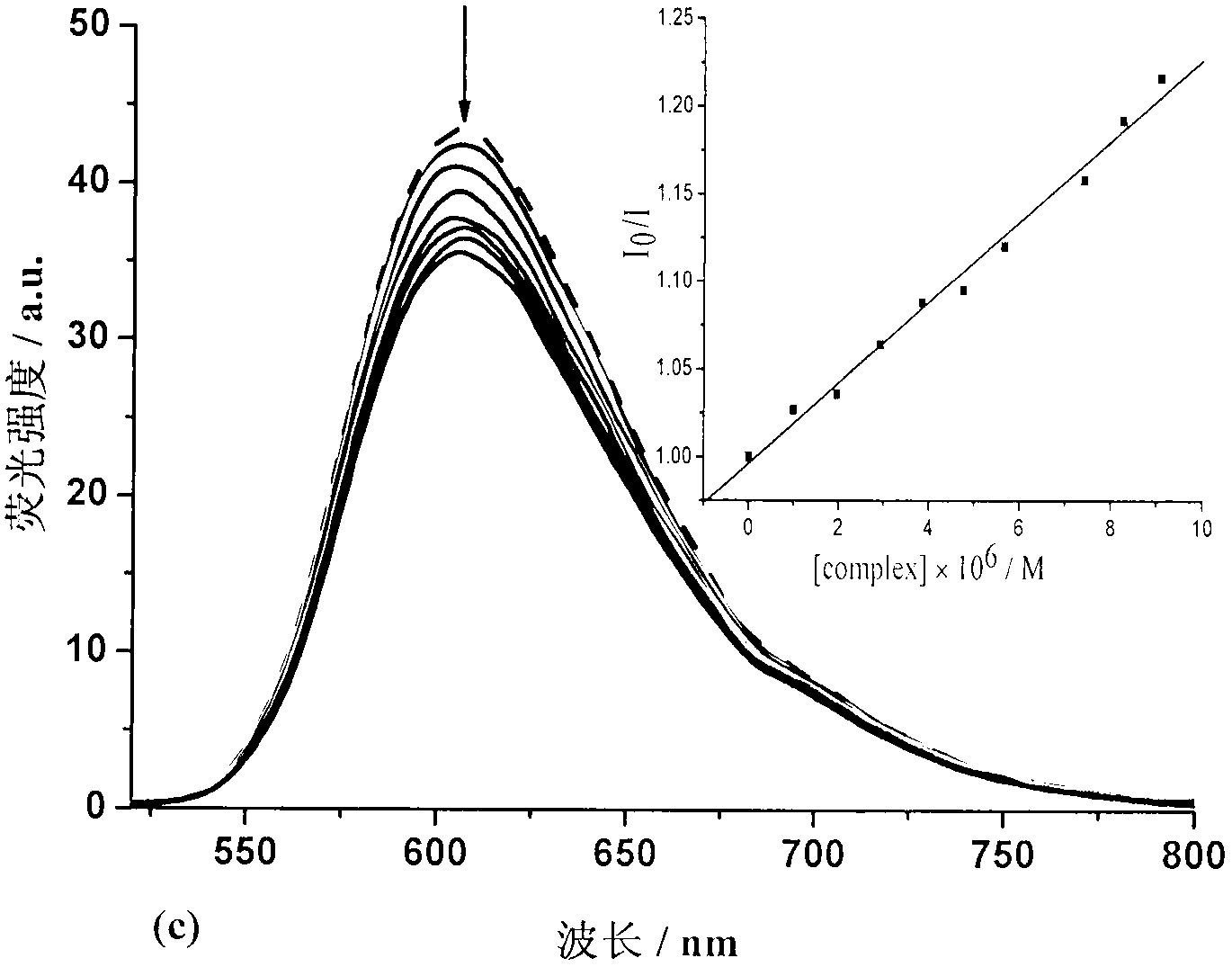
Binuclear zinc complex and preparation method and applications thereof
A zinc complex and coordination technology, applied in zinc organic compounds, drug combinations, antitumor drugs, etc., can solve the problem that the number of bases specifically recognized by natural nucleases cannot meet the rapid growth, and achieve good bonding and The effect of cutting effect, good anticancer activity, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention is described in detail as follows with reference to embodiment:
[0022] Synthesis of the main ligand L xHClO4:
[0023] Dissolve 5.83g (35.5mmol) of 2-chloromethylpyridine hydrochloride in 7mL of distilled water and keep stirring, slowly add 1.48g (7.46mmol) of 4,4'-diaminodiphenylmethane in 15mL of ethanol solution, wait After the two were mixed evenly, 2.37g (59.3mmol) NaOH 7mL aqueous solution was slowly dropped into the system, reacted at room temperature and protected from light for two weeks, stopped stirring, the solution was layered, and the lower layer was a black-red oily substance. The above reaction solution was extracted with 3x20mL dichloromethane, the organic layer solution was collected, dried with anhydrous MgSO4 for 1 hour, and the solvent was removed by rotary evaporation to obtain a red oil, which was dissolved in 80mL of absolute ethanol, and then slowly added dropwise to concentrated Perchloric acid produces yellow-brown L·x...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com