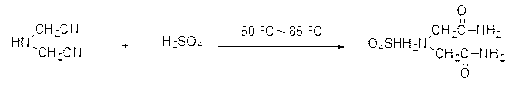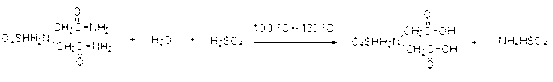Environmentally-friendly clean production method of iminodiacetic acid
A technology of iminodiacetic acid and iminodiacetonitrile is applied in the synthesis field of iminodiacetic acid, and can solve the problems of affecting the recycling of mother liquor, large amount of mother liquor, and high raw material price, avoiding corrosiveness and pollution of operating environment, Reduce water consumption and improve product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
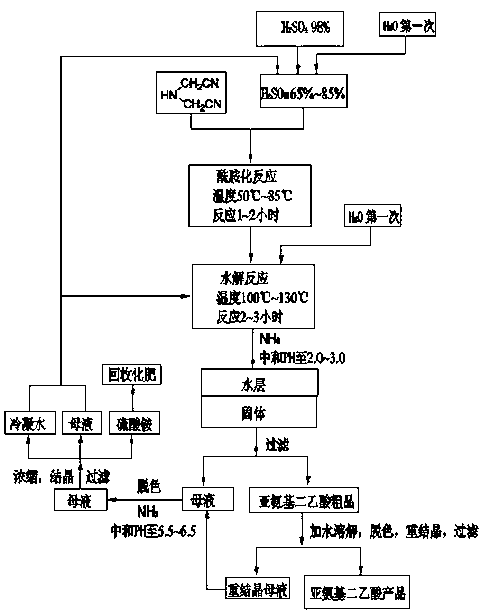
[0032] 1) In a 2000ml four-necked round bottom reaction flask, add 92 grams of water, slowly add 300 grams of 98% concentrated sulfuric acid dropwise under stirring, dilute the concentrated sulfuric acid to a mass concentration of 75%, and then cool the diluted sulfuric acid to 75°C, then slowly add 100 grams of iminodiacetonitrile with a content of 95%, and keep the feeding time for more than 30 minutes. After adding iminodiacetonitrile, continue to keep warm and stir for 2 hours. Detect the amount of iminodiacetonitrile to generate iminodiacetamide sulfate;
[0033] 2) Add 196 grams of water to the reaction system in step 1), further dilute the sulfuric acid in the reaction system to a mass concentration of 50%, then raise the temperature to 130°C, and react for 3 hours to generate iminodiacetic acid;
[0034] 3) Cool to 40°C, pass ammonia gas into the reaction system of step 2), neutralize the pH to 2.5, cool to 5°C to crystallize, separate the crystals and mother liquor, t...
Embodiment 2
[0038] 1) In a 2000ml four-necked round bottom reaction flask, add 120 grams of condensed water and mother liquor obtained in Example 1, slowly add 300 grams of 98% concentrated sulfuric acid dropwise under stirring, and dilute the concentrated sulfuric acid to a mass concentration of 70% , then cool the diluted sulfuric acid to 80°C, then slowly add 100 grams of iminodiacetonitrile with a content of 95%, keep the addition time for more than 30 minutes, after adding iminodiacetonitrile, continue to keep warm and stir for 2 hours , the amount of iminodiacetonitrile can be detected by high performance liquid chromatography in the middle to generate iminodiacetamide sulfate;
[0039] 2) Add 196 grams of condensed water and mother liquor obtained in Example 1 to the reaction system in step 1), further dilute the sulfuric acid in the reaction system to a mass concentration of 48%, then raise the temperature to 130°C, and react for 3 hours to form iminodiacetic acid;
[0040] 3) Co...
Embodiment 3
[0044] 1) In a 2000ml four-necked round bottom reaction flask, add 152 grams of condensed water and mother liquor obtained in Example 2, slowly add 300 grams of 98% concentrated sulfuric acid dropwise under stirring, and dilute the concentrated sulfuric acid to a mass concentration of 65% , then cool the diluted sulfuric acid to 75°C, then slowly add 100 grams of iminodiacetonitrile with a content of 95%, and keep the feeding time for more than 30 minutes. After adding iminodiacetonitrile, continue to keep warm and stir for 2 hours , the amount of iminodiacetonitrile can be detected by high performance liquid chromatography in the middle to generate iminodiacetamide sulfate;
[0045] 2) Add 136 grams of condensed water and mother liquor obtained in Example 2 to the reaction system in step 1), further dilute the sulfuric acid in the reaction system to a mass concentration of 50%, then raise the temperature to 120°C, and react for 3 hours to form iminodiacetic acid;
[0046] 3)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com