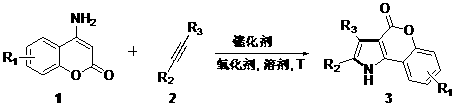Synthetic method of coumarin-pyrrole compound
A synthetic method and compound technology, applied in the field of synthesis of coumarin-pyrrole compounds, can solve the problems of low utilization rate of starting materials, limited use range, harsh reaction conditions, etc., to promote deep-level expansion and not harsh storage conditions , stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] coumarins 1a (0.2 mmol), disubstituted acetylenes 2a (0.6 mmol), palladium acetate (0.02 mmol) and copper acetate (0.04 mmol) were added to DMSO (2 mL). The reaction mixture was stirred at normal temperature and pressure under an oxygen atmosphere for 72 hours. After the completion of the reaction monitored by TLC, the organic solvent was distilled off under reduced pressure to obtain the crude product, and the product was obtained by flash column chromatography (ethyl acetate: n-hexane = 1:4) 3aa (90%). 1 H NMR (500 MHz, d 6 -DMSO, 298 K): δ 12.80 (s, 1H), 8.37–8.17 (m, 1H), 7.56–7.20 (m, 13H). 13 C NMR (125 MHz, d 6 -DMSO, 298 K): δ 157.74, 151.61, 135.78, 133.88, 133.55, 131.49, 131.07, 129.24, 128.82, 128.18, 128.09, 127.14, 124.39, 122.11, 121.09, 117.00, 113.86, 107.29, 40.73, 40.46, 40.18, 39.90, 39.62, 39.35, 39.07. HRMS (ESI): Calculated [C 23 h 15 NO 2 +H] + 338.1176, actual value 338.1177.
Embodiment 2
[0029]
[0030] The operation steps are the same as in Example 1, and the yield is 89%. 1 H NMR (500 MHz, d 6 -DMSO, 298 K): δ 12.72 (s, 1H), 8.11 (s, 1H), 7.65–7.08 (m, 12H), 2.43 (s, 3H). 13 C NMR (125 MHz, d 6 -DMSO, 298 K): δ 157.87, 149.80, 135.82, 133.73, 133.61, 133.49, 131.48, 131.06, 130.09, 128.80, 128.70, 128.13, 128.10, 127.14, 121.91, 121.06, 116.77, 113.52, 107.32, 40.73, 40.46, 40.18, 39.90, 39.62, 39.35, 39.07, 20.99. HRMS (ESI): Calculated [C 24 h 17 NO 2 -H] - 350.1187, actual value 350.1197.
Embodiment 3
[0032]
[0033] The operation steps are the same as in Example 1, and the yield is 99%. 1 H NMR (500 MHz, d 6-DMSO, 298 K): δ 12.75 (s, 1H), 8.16 (s, 1H), 7.65–6.92 (m, 12H), 2.74 (q, J = 7.6 Hz, 2H), 1.30 (t, J = 7.6 Hz, 3H). 13 C NMR (125 MHz, d 6 -DMSO, 298 K): δ 158.01, 150.04, 139.93, 136.07, 133.85, 133.70, 131.62, 131.17, 129.18, 128.93, 128.85, 128.27, 128.22, 127.25, 121.15, 120.79, 116.91, 113.68, 107.36, 40.73, 40.46, 40.18, 39.90, 39.62, 39.35, 39.07, 28.18, 15.89. HRMS (ESI): Calculated [C 25 h 19 NO 2 -H] - 364.1343, actual value 364.1346.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com