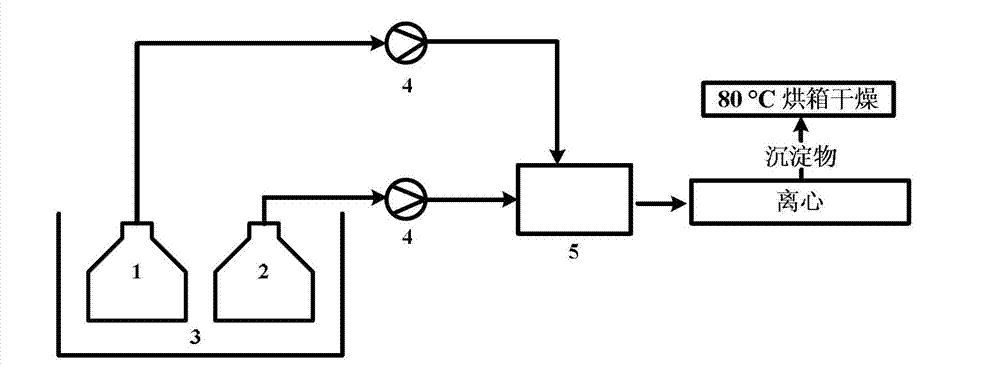
Method for continuously and controllably preparing small-size pyrithione granules
A technology of pyrithione salt and pyrithione, which is applied in chemical instruments and methods, chemical/physical/physical chemical processes, organic chemistry, etc., to achieve the effects of simple production process and device, good permeability and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
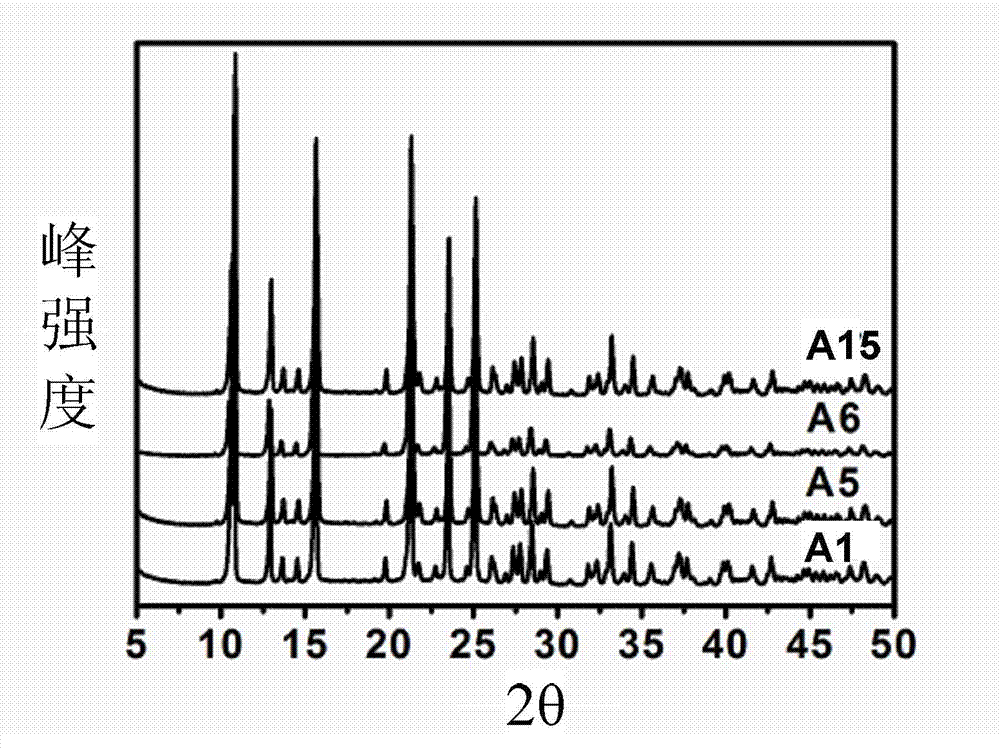
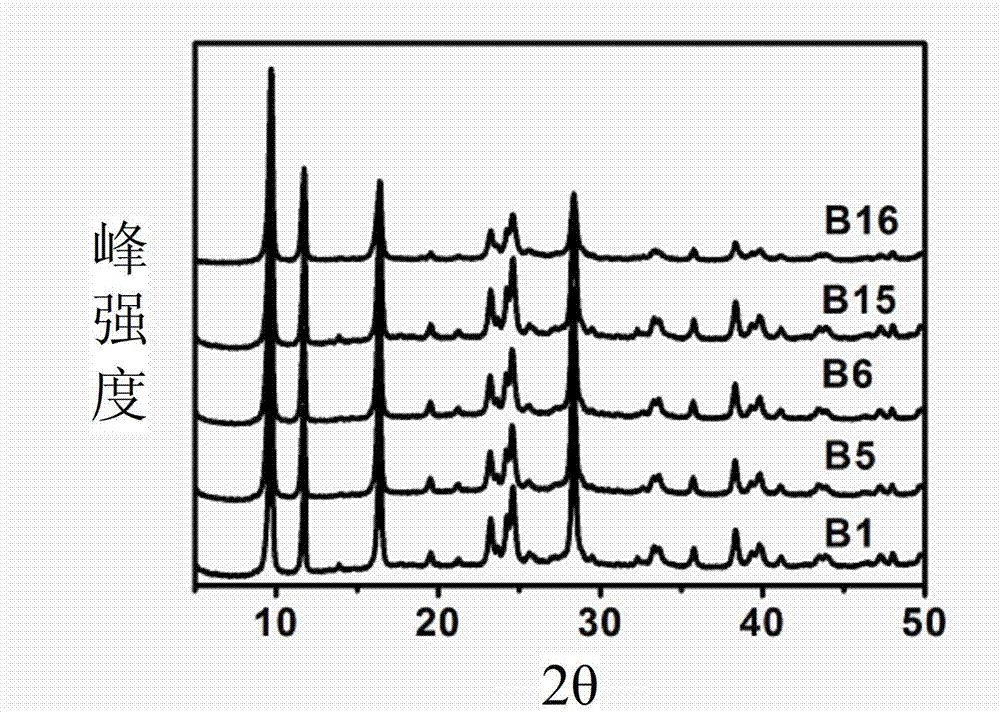
[0030] Using 0.2mol / L zinc nitrate solution, under the condition that the flow rates of zinc nitrate and sodium pyrithione solution are both 15mL / min, the two are respectively injected into a micro-mixer with an inner diameter of 500 μm and a mixing channel length of 2cm. Preparation of Zinc Pyrithione by Mixed Reaction. Control the reaction temperature to be 80°C, and the molar ratio of raw materials R=2 (R≡n(sodium pyrithione) / n(Zn 2+ )), the corresponding concentration of pyrithione sodium solution is 0.4mol / L. The obtained samples were centrifuged and washed 2-3 times with deionized water, and dried overnight in an oven at 80°C. The sample is denoted as A1. From the scanning electron micrograph of the sample, it was found that the obtained sample was an irregular block (such as Figure 4 ). The average particle size of the sample is 0.48 μm and the particle size distribution is 0.27-0.79 μm according to the electron microscope photos.
example 2-4
[0032]The experiment was carried out in the same manner as Example 1, but the reaction temperature was changed. The reaction temperatures are 20°C, 40°C, and 60°C, respectively, and the zinc pyrithione obtained sequentially is A2, A3, and A4. According to the scanning electron microscope photos of the product, the average particle size and particle size distribution of A2 are 0.90 μm and 0.25-1.99 μm, the average particle size and particle size distribution of A3 are 0.60 μm and 0.22-1.2, and the average particle size distribution of A4 The diameter and particle size distribution are 0.52 μm and 0.2-1.00 μm, respectively. It can be seen that with the increase of reaction temperature, the average particle size of the product decreases and the distribution narrows.
example 5-6
[0034] Experiments were carried out in the same manner as in Example 1, but the raw material molar ratio R was changed. Keep the concentration of zinc nitrate solution at 0.2mol / L, change the R value to 1 and 4 respectively, and the zinc pyrithione obtained in turn is A5, A6. According to the scanning electron microscope photos of the product, the average particle size and particle size distribution of A5 are 0.55 μm and 0.29-0.85 μm, respectively, and the average particle size and particle size distribution of A6 are 0.53 μm and 0.23-0.75 μm, respectively. It can be seen that the R value has little effect on the particle size of the product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com