Bacteria nitroreductase detection kit and special-purpose fluorescence probe thereof
A technology of nitroreductase and reductase, which is applied in the fields of fluorescence/phosphorescence, luminescent materials, organic chemistry, etc., to achieve the effects of high sensitivity, fast reaction speed, and easy promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
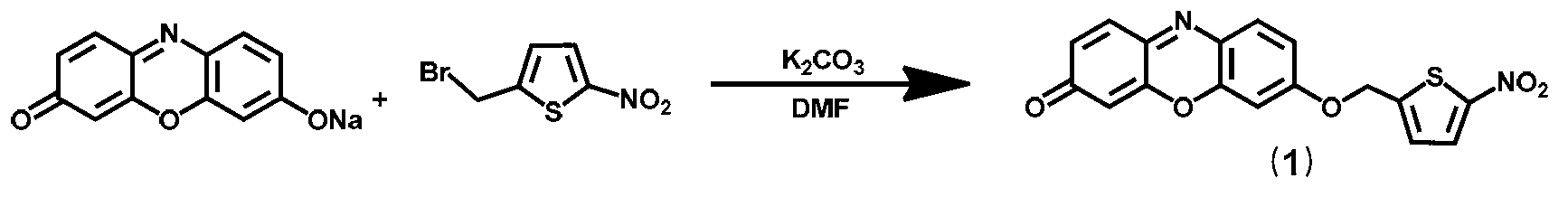
[0042] Example 1, preparation of 7-[(5-nitrothiophen-2-yl)methoxy]-3H-phenoxazin-3-one shown in formula I
[0043] according to figure 1 The chemical reaction scheme shown in the preparation was carried out.
[0044] The operation steps are as follows: Dissolve 7-hydroxyphenoxazinone sodium salt (235mg, 1mmol) in DMF (10mL), add 2-bromomethyl-5-nitrothiophene (223mg, 1mmol), catalyst potassium carbonate (210mg , 1 mmol) in the reaction solution, and reacted at 20°C for 6 hours. After completion of the reaction, after cooling, the solvent was concentrated under reduced pressure to obtain a crude product, which was purified by column chromatography using n-hexane / ethyl acetate (2:1, v / v) as eluent to obtain 180 mg of the product.
[0045] The structural characterization data results of the product are as follows:
[0046] 1 H NMR (600MHz, 298K, CF 3COOD): δ8.40(d, J=9.4Hz, 1H), 8.36(d, J=9.4Hz, 1H), 7.92(d, J=4.2Hz, 1H), 7.69(t, J=2.8Hz, 1H), 7.67(t, J=2.8Hz, 1H), 7.56(d, ...
Embodiment 2
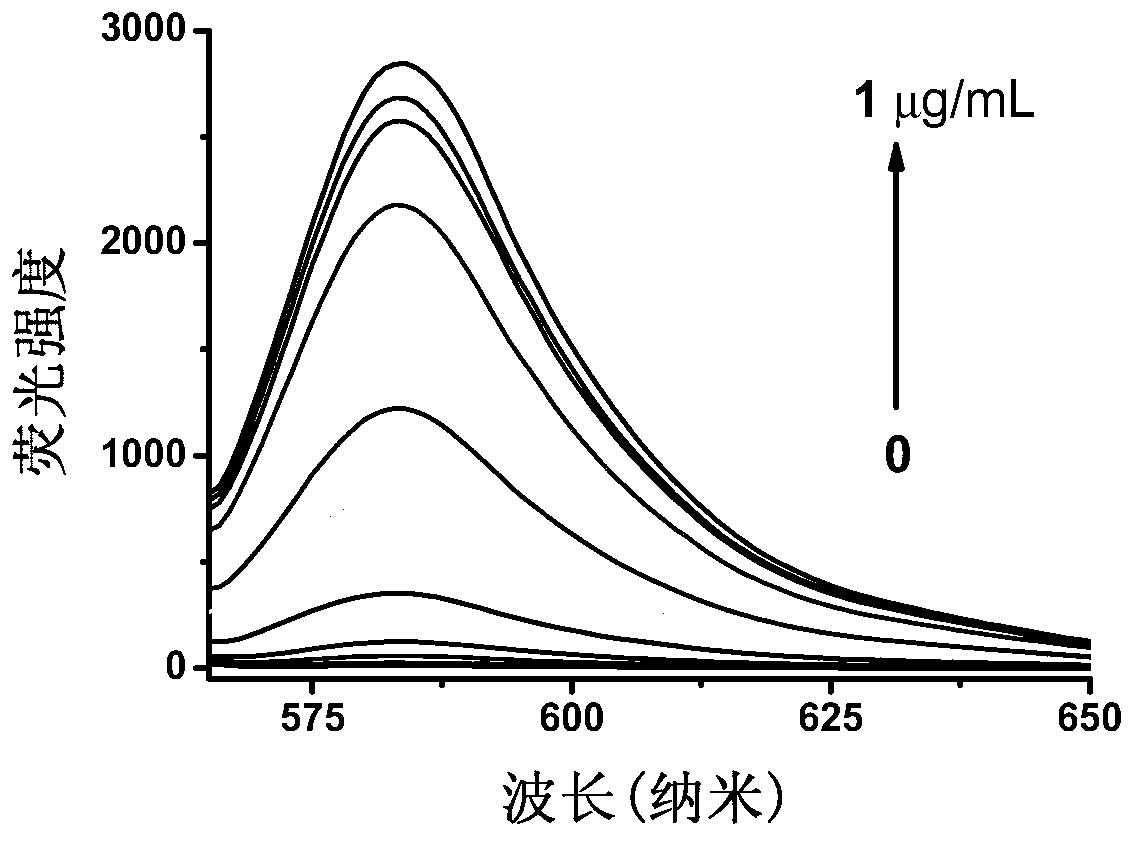
[0050] Embodiment 2: Spectral properties of reagent 1 (i.e. compound shown in formula I) reacting with different concentrations of nitroreductase
[0051] Weigh 3.38 mg of reagent 1 and make 10 ml of dimethyl sulfoxide solution as mother solution (1 mM).
[0052] Add 50 μl of the above mother solution dropwise to a certain amount of 10 mM phosphate buffer solution containing 50 μM coenzyme (nicotinamide adenine dinucleotide), then add different concentrations of nitroreductase solutions, and then use 10 mM phosphate buffer The solution was adjusted to 10ml. After reacting at 37° C. for 20 min, measure its UV-Vis absorption spectrum and fluorescence emission spectrum. When measuring the fluorescence emission spectrum, de-excite at 550nm; the slit width for excitation and emission is 10nm; the voltage is 400V.
[0053] figure 2 It is the fluorescence spectrum of reagent 1 reacting with 0-1 μg / ml nitroreductase.
[0054] figure 2 The results show that reagent 1 in the pres...
Embodiment 3
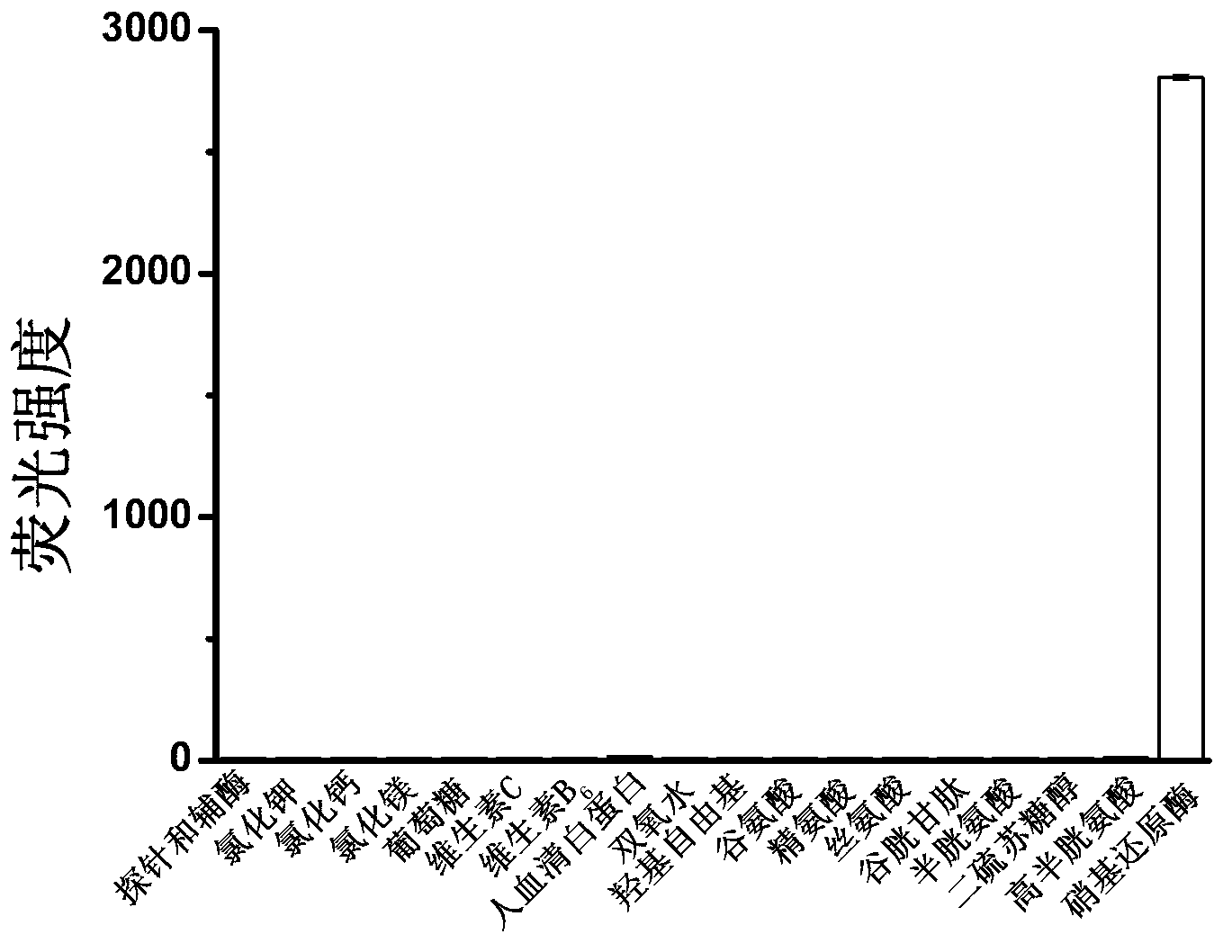
[0058] Embodiment 3: Reagent 1 (compound shown in formula I) reacts with other species (selectivity research)
[0059] Add various substances to the 5μM reagent 1 solution: potassium chloride (150mM), calcium chloride (2.5mM), magnesium chloride (2.5mM), glucose (10mM), vitamin C (1mM), vitamin B 6 (1mM), human serum albumin (100μM), hydrogen peroxide (10μM), hydroxyl radical (10μM), glutamic acid (1mM), arginine (1mM), serine (1mM), glutathione (5mM) , cysteine (1 mM), homocysteine (1 mM), dithiothreitol (1 mM) and nitroreductase (0.5 μg / mL). After reacting at 37°C for 20 min, the fluorescence emission spectrum was measured. When measuring the fluorescence emission spectrum, de-excite at 550nm; the slit width for excitation and emission is 10nm; the voltage is 400V.
[0060] 50 μl of reagent 1 stock solution (1 mM) was added to 10 ml of the solution mixed with the above-mentioned substances, and nitroreductase was added to make the final concentration 0.5 μg / mL.
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com