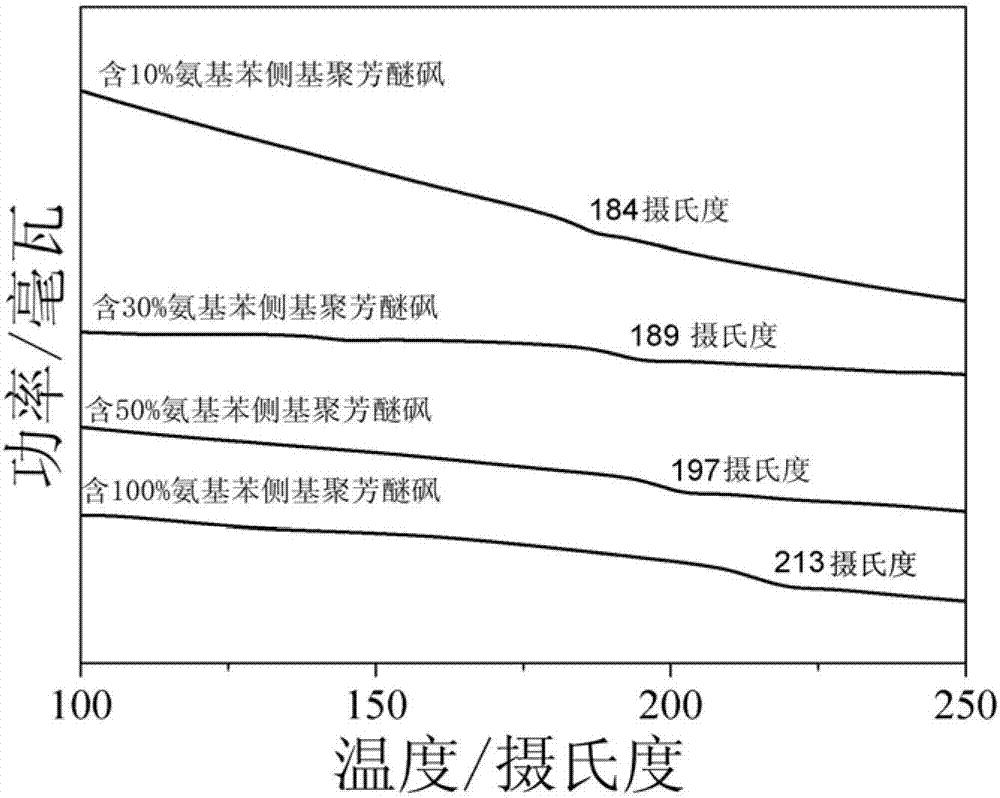
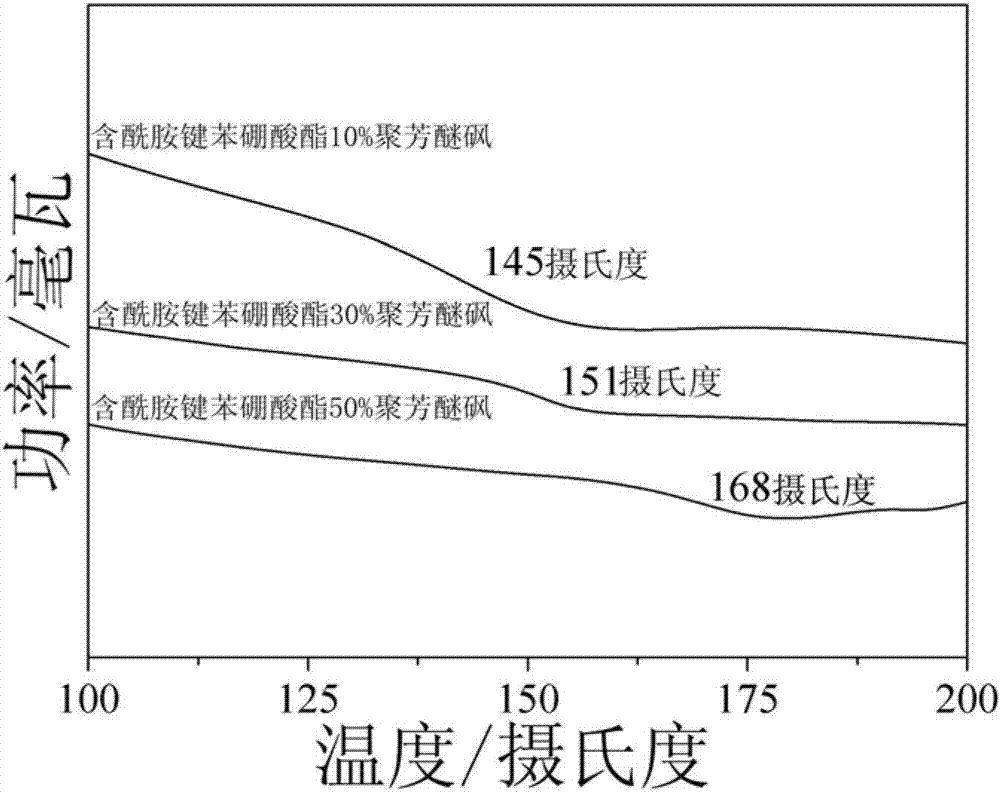
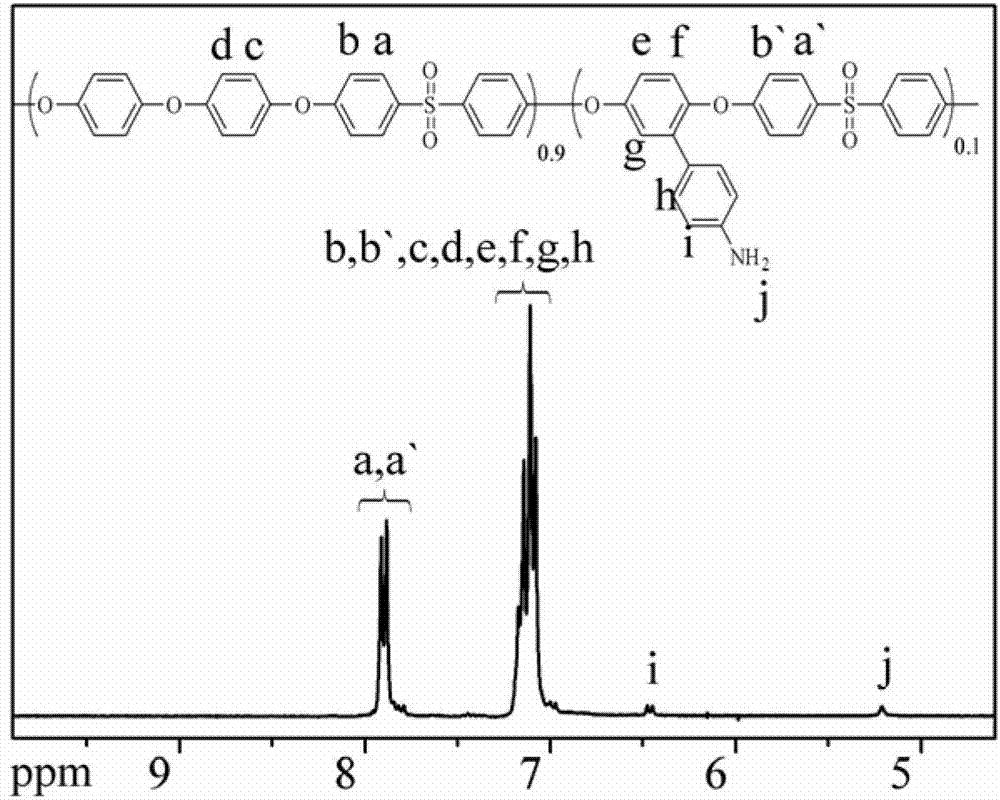
Amido bond phenylboronic acid ester-containing polyether sulphone polymer and preparation method thereof
A technology of bond phenyl boronate and polyaryl ether sulfone is applied in the field of amide bond-containing phenyl boronate polyaryl ether sulfone polymer and its preparation, which can solve the problem that the position and content of boron-containing polymer cannot be introduced, and achieves The method is simple and easy to implement, the grafting rate is high, and the effect of excellent high temperature ablation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of polyarylethersulfone containing p-aminophenyl side groups (1)
[0047] 5.4597g (0.027mol) 4,4`-dihydroxydiphenyl ether, 0.6037g (0.003mol) 2-(4`-aminophenyl)-1,4-hydroquinone monomer, 7.6275g (0.03 mol) 4,4`-difluorodiphenylsulfone monomer and 4.5603g of anhydrous potassium carbonate, 57mL of sulfolane, 20mL of toluene, put into a three-necked flask equipped with a nitrogen hole, mechanical stirring and a water device, and nitrogen , start stirring, heat up to the reflux of the azeotropic dehydrating agent, react for 2 hours, remove the azeotropic dehydrating agent, heat up to 170°C and continue the reaction for 8 hours; then the obtained polymer solution is precipitated in deionized water, pulverized, washed , and dried to obtain 12 g off-white polyarylethersulfone polymer powder containing p-aminophenyl side groups.
[0048] The molecular formula is as follows,
[0049]
[0050] where n is 14.
Embodiment 2
[0051] Example 2: Amidation reaction of polyarylethersulfone containing p-aminobenzene side groups and p-carboxyphenylboronic acid pinacol ester to prepare polyarylethersulfone material containing amide bond phenylboronic acid ester (1)
[0052] Put 4.16g of polyaryl ether sulfone containing p-aminophenyl side groups and 0.27g of p-carboxyphenylboronic acid pinacol ester (1.1 times the molar weight of amino groups in polyaryl ether sulfone containing aminophenyl side groups) into a stirring Add 0.31g of N,N-dicyclohexyldiimine (DCC) (1.5 times the molar amount of amino groups in polyarylethersulfone containing aminophenyl side groups), 0.18g of 4-dimethylaminopyridine (DMAP) (0.15 times the molar weight of amino groups in polyarylethersulfone containing aminobenzene side groups), then vacuumize and release high-purity nitrogen, then vacuumize and release high-purity nitrogen, repeat 4 times, and finally inject 100mL tetrahydrofuran to dissolve, and then Seal, start and stir, a...
Embodiment 3
[0053] Example 3: Preparation of polyarylethersulfone containing p-aminobenzene side groups (2)
[0054] 4.2464g (0.009mol) 4,4`-dihydroxydiphenyl ether, 1.8110g (0.021mol) 2-(4`-aminophenyl)-1,4-hydroquinone monomer, 7.6275g (0.03 mol) 4,4`-difluorodiphenyl sulfone monomer and 4.5603g of anhydrous potassium carbonate, 55ml of sulfolane, and 12mL of toluene were placed in a three-necked flask equipped with a nitrogen through hole, mechanical stirring and a water device, and nitrogen was passed. Start stirring, heat up to the reflux of the azeotropic dehydrating agent, react for 2 hours, get rid of the azeotropic dehydrating agent, heat up to 170°C and continue the reaction for 8 hours; then the obtained polymer solution is precipitated in deionized water, pulverized, washed, After drying, 13 g of off-white polyarylethersulfone polymer powder with p-aminophenyl side groups was obtained.
[0055] The molecular formula is as follows:
[0056]
[0057] where n=13.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com