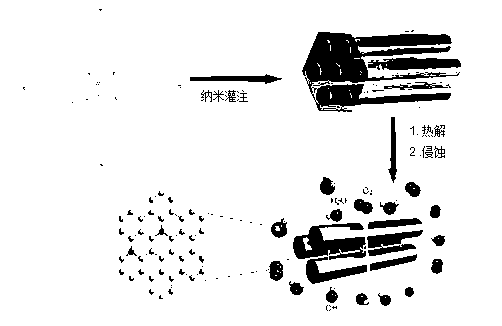
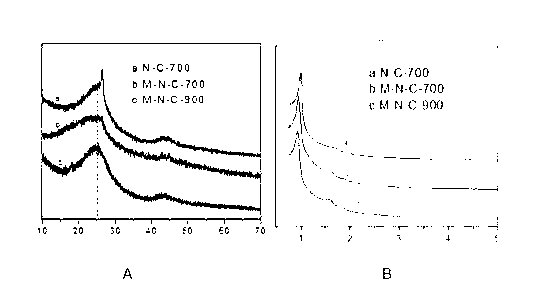
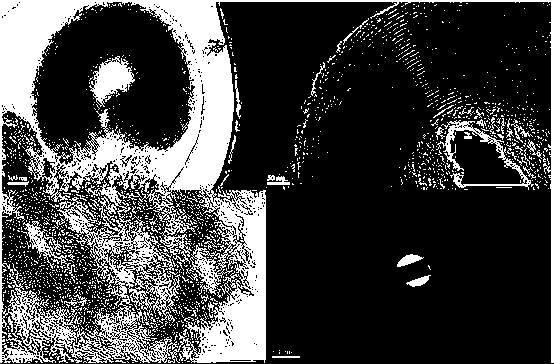
Preparation method of ordered mesoporous non-noble-metal-nitrogen-graphitized carbon material
A non-precious metal and graphitized carbon technology, applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve problems such as reducing the optimal amount of metal used, reducing the nitrogen density of materials, etc., to achieve superior catalysis Effects of stability and methanol resistance, good electrical conductivity, and good dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] a. Synthesis of Metalloporphyrins
[0034] Add 4 g of tetrapyridyl porphyrin and 1.54 g of cobalt chloride into 150 mL of absolute ethanol, reflux at 50° C. for 6 h, and ethanol recrystallize.
[0035] b. Synthesis of filling materials
[0036] Add 1g of cobalt porphyrin to a mixed solution of 10mL of glacial acetic acid and 10mL of deionized water, stir to dissolve, add 1g of two-dimensional mesoporous silica SBA-15, and keep stirring until dry at room temperature; then, keep the temperature at 60°C After drying, the metal complex is filled into the mesoporous channel material.
[0037] c. High temperature roasting of filling materials
[0038] The obtained filling material was placed in a quartz tube, heated at 600°C in a 40ml / min pure nitrogen atmosphere, and kept for 3 hours to carbonize the filling material to obtain a black powder.
[0039] d. Removal of hard template mesoporous silica
[0040] The black powder prepared above was stirred with 25% hydrofluoric ...
Embodiment 2
[0042] a. Synthesis of Metalloporphyrins
[0043] Add 4g of tetrapyridyl porphyrin and 1.05g of anhydrous ferric chloride into 150mL of absolute ethanol, reflux at 55°C for 6h, and recrystallize the ethanol.
[0044] b. Synthesis of filling materials
[0045] Add 1g of iron porphyrin to 10mL of glacial acetic acid and 20mL of deionized water mixed solution, stir to dissolve, add 0.5g of two-dimensional mesoporous silica SBA-15, and keep stirring until dry at room temperature; then, keep the temperature at 60°C After drying, the metal complex is filled into the mesoporous channels.
[0046] c. High temperature roasting of filling materials
[0047] The obtained filling material was placed in a quartz tube, heated at 700° C. in a 40 ml / min pure nitrogen atmosphere, and kept for 3 hours to carbonize the filling material to obtain a black powder.
[0048] d. Removal of hard template mesoporous silica
[0049] The black powder prepared above was stirred with 25% hydrofluoric ac...
Embodiment 3
[0051] a. Synthesis of Metalloporphyrins
[0052] Add 4g of tetrapyridyl porphyrin and 1.02g of chromium chloride into 150mL of absolute ethanol, reflux at 55°C for 7h, and recrystallize from ethanol.
[0053] b. Synthesis of filling materials
[0054] Add 1g of chromium porphyrin to a mixed solution of 10mL of glacial acetic acid and 30mL of deionized water, stir to dissolve, add 0.35g of two-dimensional mesoporous silica SBA-15, and keep stirring until dry at room temperature; then, at 60°C Dry at constant temperature to obtain metal complexes to fill the mesoporous channels.
[0055] c. High temperature roasting of filling materials
[0056] The obtained filling material was placed in a quartz tube, heated at 700°C in a 40ml / min pure nitrogen atmosphere, and kept for 4 hours to carbonize the filling material to obtain a black powder.
[0057] d. Removal of hard template mesoporous silica
[0058] Stir the 25% hydrofluoric acid solution for the black powder prepared ab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com