Adefovir dipivoxil composition and preparation method thereof
A technology of adefovir dipivoxil and prescription, which is applied in the field of adefovir dipivoxil tablets and its preparation, can solve the problems of poor stability of adefovir dipivoxil tablets, and achieve improved anti-hepatitis B, fewer procedures, and blood drug concentration increased effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0042] Implementation example 1
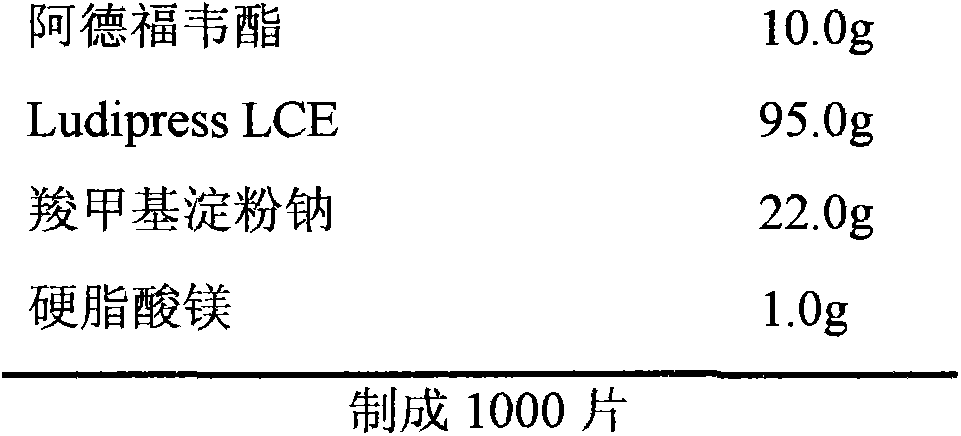
[0043] Every 1000 adefovir dipivoxil tablets, weigh materials according to the following formula:
[0044] Tablet prescription:
[0045]
[0046] Coating Solution Prescription:
[0047]
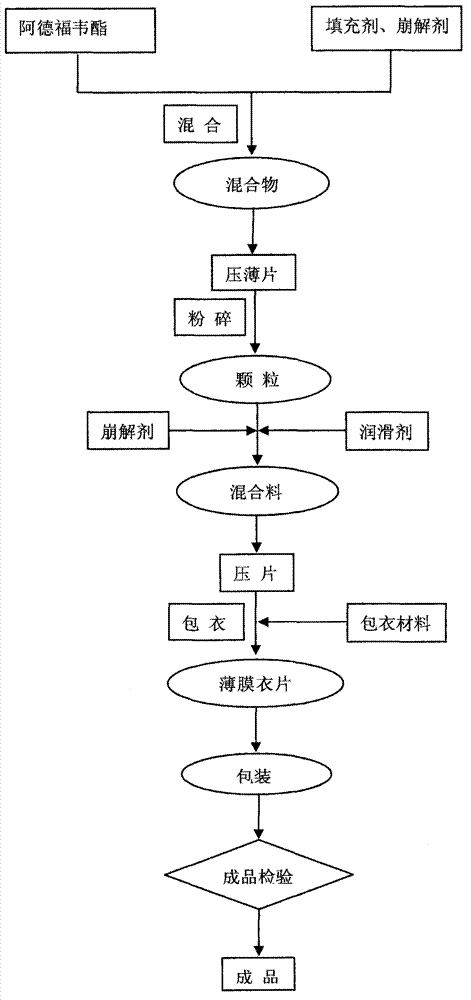
[0048] making process:
[0049]1. Grind adefovir dipivoxil with an ultrafine pulverizer until the average particle size is less than 50 μm, pass through a 60-mesh sieve for Ludipress LCE and sodium carboxymethyl starch respectively, and set aside;
[0050] 2. Weigh the prescribed amount of adefovir dipivoxil and sodium carboxymethyl starch into the wet mixing granulator, set the stirring speed to 450r / min, the chopping speed to 1200r / min, mix for 180s, and add the prescribed amount after discharging Mix Ludipress LCE for 300s, then add the prescribed amount of magnesium stearate and mix for 50s;
[0051] 3. Take the mixture in step 2 to measure the fluidity of the powder (indicated by the angle of repose θ), moisture and the content of the main drug...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com