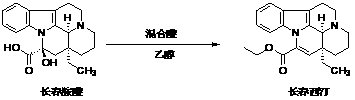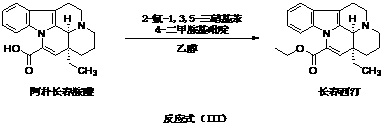Preparation method of vinpocetine
A technology of vinpocetine and vincristine, which is applied in the direction of organic chemistry, etc., can solve the problems that the product quality cannot meet the high-quality requirements of raw materials, harsh reaction conditions, and high toxicity of reagents, and can meet the requirements of quality, stable quality and operation. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 The preparation method of vinpocetine
[0036] The preparation method of present embodiment Vinpocetine, comprises the steps:
[0037] 1) Add 10 grams of concentrated sulfuric acid and 5 grams of vinblastine to 100 grams of absolute ethanol. After stirring and dissolving, slowly add 10 grams of thionyl chloride dropwise at 0-10°C, heat to 80°C, and react for 24 hour, the reaction solution was prepared;
[0038] 2) Concentrate the reaction solution under reduced pressure, dilute the concentrated residue with 50ml of water, adjust the pH to 7-8, and extract with dichloromethane (50ml×2);
[0039] 3) After the dichloromethane extract phases were combined, they were washed with 50 ml of saturated brine, dried over anhydrous magnesium sulfate, filtered, the filtrate was concentrated under reduced pressure at 40°C, and the concentrated residue was recrystallized with absolute ethanol to obtain 3.5 g of Vinpocetine .
[0040] The melting point of V...
Embodiment 2-13
[0041] Example 2-13 The preparation method of vinpocetine
[0042] The preparation method of Vinpocetine described in embodiment 2-13, comprises the steps:
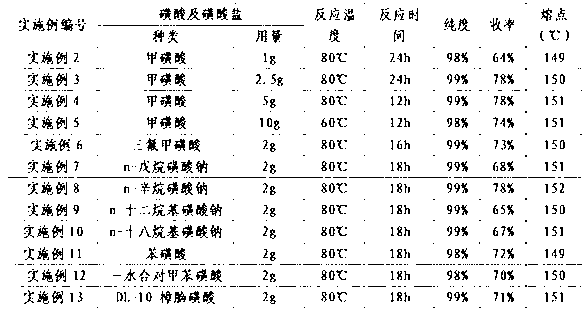
[0043] 1) Add 10 grams of concentrated sulfuric acid, 5 grams of vinblastine and the sulfonic acid or sulfonate described in Table 1 to 100 grams of absolute ethanol, stir and dissolve, heat to the temperature conditions described in Table 1, and react 12 to 24 hour, the reaction solution was prepared;
[0044] 2) Concentrate the reaction solution under reduced pressure, dilute the concentrated residue with 50ml of water, adjust the pH to 7-8, and extract with dichloromethane (50ml×2);
[0045] 3) After the dichloromethane extracts were combined, they were washed with 50 ml of saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure at 40°C, and the concentrated residue was recrystallized with absolute ethanol to obtain Vinpocetine.
[004...
Embodiment 14-16
[0049] Examples 14-16 The preparation method of vinpocetine
[0050] The preparation method of Vinpocetine described in embodiment 14-16, comprises the steps:
[0051] 1) Add concentrated sulfuric acid, 5 grams of vinblastine and methanesulfonic acid into absolute ethanol (the amount of the aforementioned substances is as described in Table 2), stir and dissolve, heat to 80°C, and react for 24 hours to obtain a reaction solution ;
[0052] 2) Concentrate the reaction solution under reduced pressure, dilute the concentrated residue with 50ml of water, adjust the pH to 7-8, and extract with dichloromethane (50ml×2);
[0053] 3) After the dichloromethane extracts were combined, they were washed with 50 ml of saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated under reduced pressure at 40°C, and the concentrated residue was recrystallized with absolute ethanol to obtain Vinpocetine.
[0054] The melting point, yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com