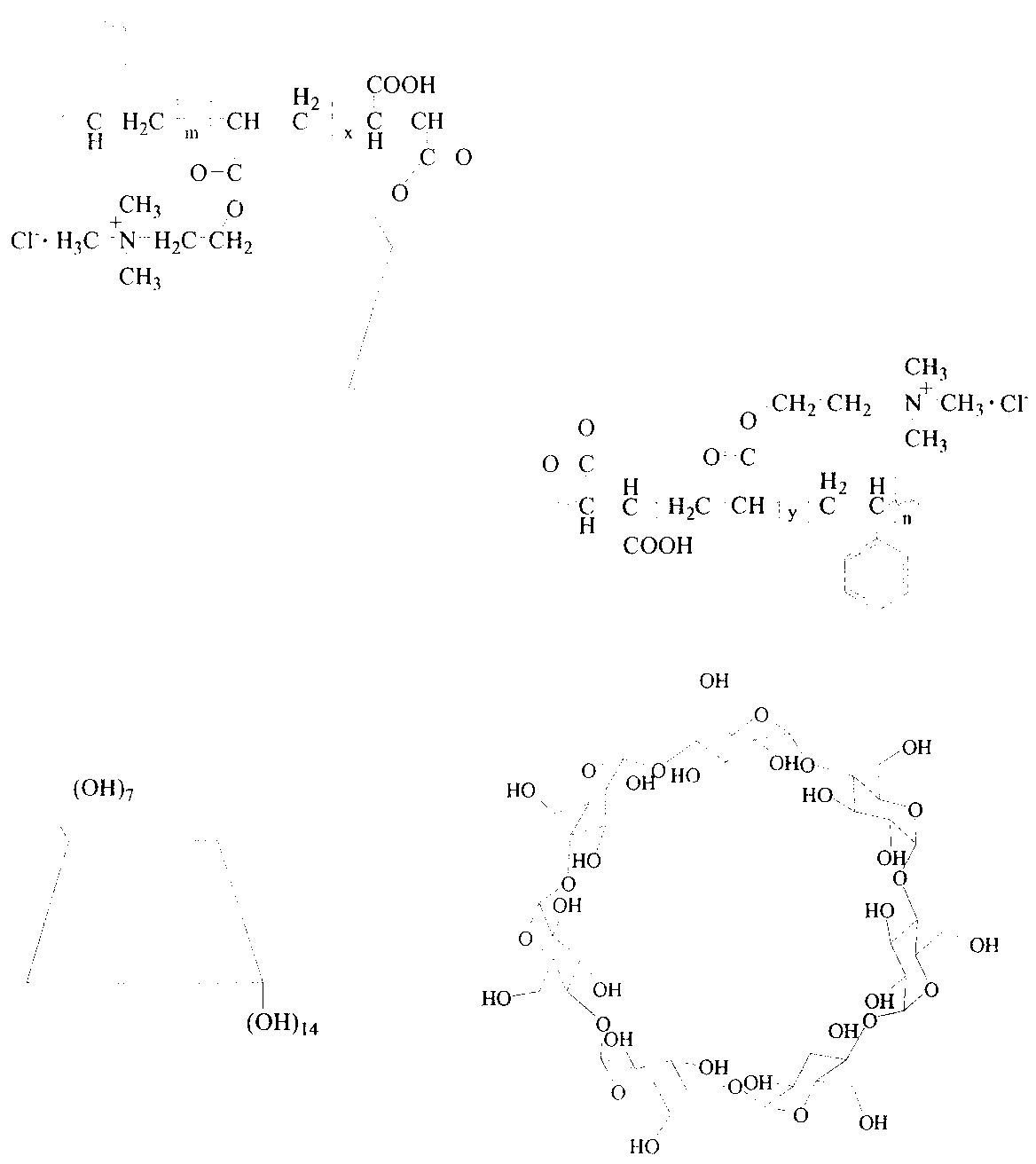Preparation method and application of hydrophobization beta-cyclodextrin cation polyelectrolyte
A cationic poly-cyclodextrin technology, applied in the direction of flocculation/sedimentation water/sewage treatment, etc., can solve problems such as difficult structure of modified products, limited modification scale, impact on flocculation performance, etc., achieves fast decolorization rate and small environmental pollution , Good salt tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
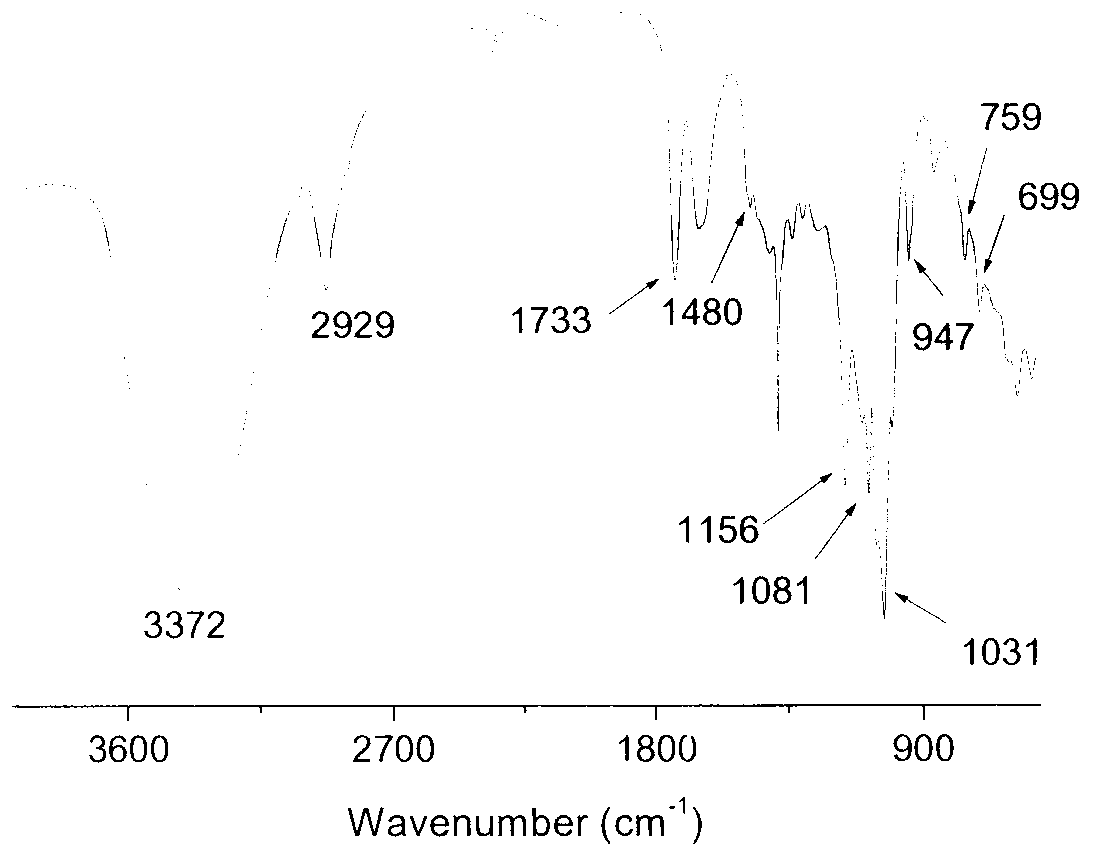
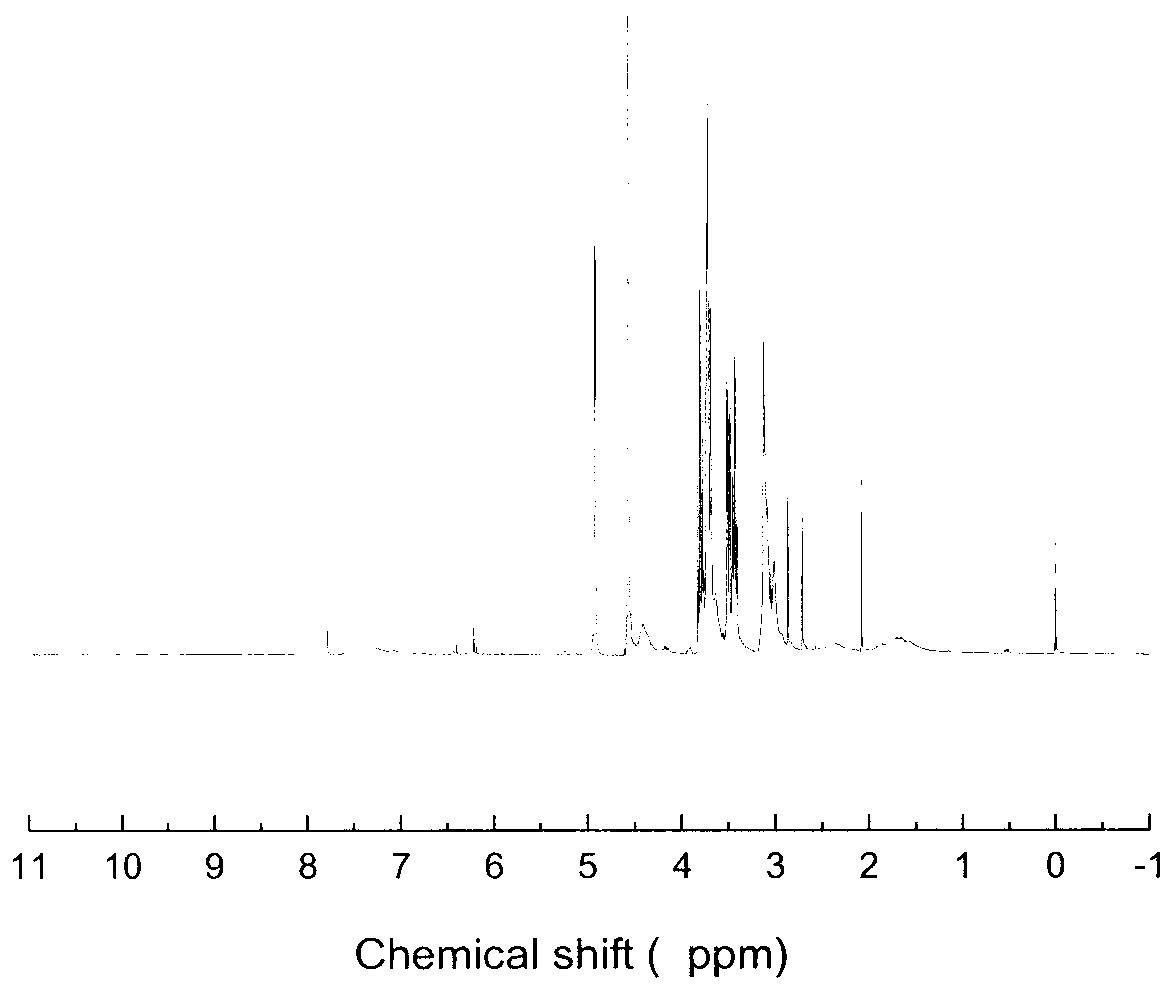
Image
Examples
Embodiment 1
[0036] (1) Add 1 part of maleic anhydride (MAH), 2.32 parts of β-CD and 33 parts of N,N-dimethylformamide (DMF) into a three-neck flask equipped with mechanical stirring and a nitrogen conduit. Stir at 80°C for 10 hours. After the reaction, cool to room temperature, add 66 parts of chloroform, and vacuum filter to obtain a white precipitate, then wash with acetone, repeat several times, and dry in a vacuum oven at 50°C for 24 hours to obtain a polymerizable β-CD- MAH.
[0037] (2) Add 45 parts of β-CD-MAH, 50 parts of DAC, 5 parts of styrene and 400 parts of deionized water into a three-neck flask equipped with magnetic stirring, condenser and nitrogen gas conduit, and pass through nitrogen. Raise the temperature to 55°C, wait until β-CD-MAH, DAC, and St are mixed evenly, and add 0.45 parts of K 2 S 2 o 8 , 0.17 part NaHSO 3 . Nitrogen flow was continued, and the reaction was incubated for 4 hours. The reaction system was cooled to room temperature, 800 parts of acetone...
Embodiment 2
[0040] (1) Add 1 part of maleic anhydride (MAH), 2.32 parts of β-CD and 33 parts of N,N-dimethylformamide (DMF) into a three-neck flask equipped with mechanical stirring and a nitrogen conduit. Stir at 80°C for 10 hours. After the reaction, cool to room temperature, add 66 parts of chloroform, and vacuum filter to obtain a white precipitate, then wash with acetone, repeat several times, and dry in a vacuum oven at 50°C for 24 hours to obtain a polymerizable β-CD- MAH.
[0041] (2) Add 50 parts of β-CD-MAH, 45 parts of DAC, 5 parts of styrene and 400 parts of deionized water into a three-neck flask equipped with magnetic stirring, condenser and nitrogen gas conduit, and pass through nitrogen. Raise the temperature to 55°C, wait until β-CD-MAH, DAC, and St are mixed evenly, and add 0.45 parts of K 2 S 2 o 8 , 0.17 part NaHSO 3 . Nitrogen flow was continued, and the reaction was incubated for 4 hours. The reaction system was cooled to room temperature, 800 parts of acetone...
Embodiment 3
[0044] (1) Add 1 part of maleic anhydride (MAH), 2.32 parts of β-CD and 33 parts of N,N-dimethylformamide (DMF) into a three-neck flask equipped with mechanical stirring and a nitrogen conduit. Stir at 80°C for 10 hours. After the reaction, cool down to room temperature, add 66 parts of chloroform, vacuum filter to obtain a white precipitate, wash with acetone, repeat several times, and dry in a vacuum oven at 50°C for 24 hours to obtain a polymerizable β-CD derivative (β-CD-MAH).
[0045] (2) Add 57 parts of β-CD-MAH, 40 parts of DAC, 3 parts of styrene and 400 parts of deionized water into a three-neck flask equipped with magnetic stirring, condenser and nitrogen gas conduit, and pass through nitrogen. Raise the temperature to 45°C, wait until β-CD-MAH, DAC, and St are mixed evenly, and add 0.45 parts of K 2 S 2 o 8 , 0.17 part NaHSO 3 . Nitrogen flow was continued, and the reaction was incubated for 4 hours. The reaction system was cooled to room temperature, 800 par...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com