Docetaxel freeze-dried microemulsion preparation and preparation method thereof
A technology of docetaxel and emulsion preparations, applied in the directions of freeze-drying delivery, pharmaceutical formulations, emulsion delivery, etc., can solve the problems of small allergic reactions, toxic and side effects, and achieve small allergic reactions, no vascular irritation, and fluidity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-16
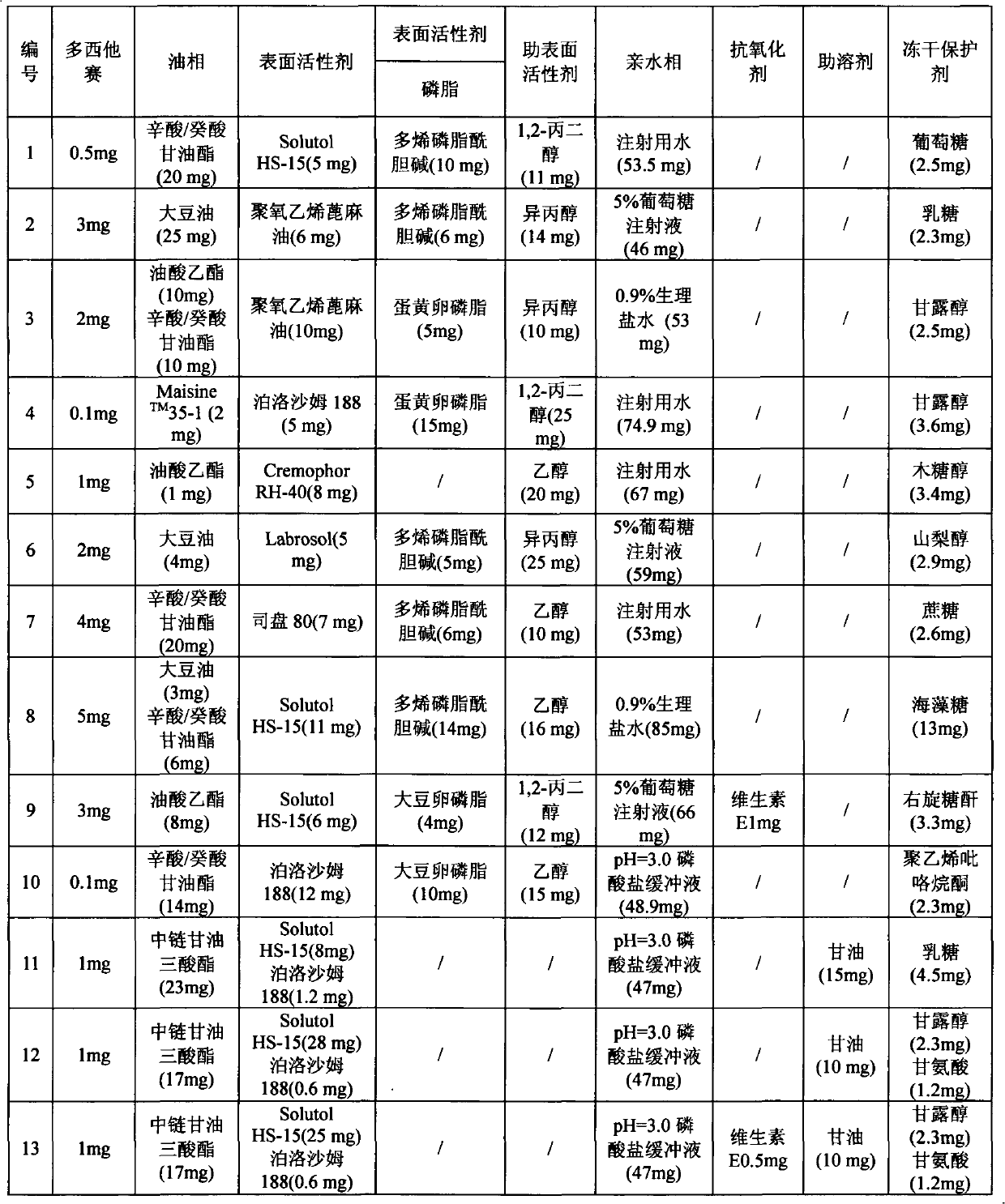
[0030]The raw material ratio of Examples 1-16 is shown in Table 1 below.
[0031] Table 1
[0032]
[0033]
Embodiment 1
[0035] Preparation of lyophilized microemulsion of docetaxel: 5 mg Solutol HS-15, 20 mg caprylic acid / capric glyceride and 10 mg polyene phosphatidylcholine were heated and dissolved in 11 mg 1,2-propanediol in a water bath at 40 ° C, and the After dissolving, add 0.5 mg of docetaxel; then slowly add 53.5 mg of water for injection (containing 2.5 mg of glucose), stir while adding, filter to obtain a transparent microemulsion with opalescence, lyophilize to remove water, and obtain a lyophilized microemulsion , freeze-dried to remove moisture, and obtained freeze-dried microemulsion.
[0036] Preparation of the docetaxel lyophilized microemulsion dilution: the obtained lyophilized microemulsion was redissolved with 0.9% physiological saline to a docetaxel concentration of 0.7 mg / mL.
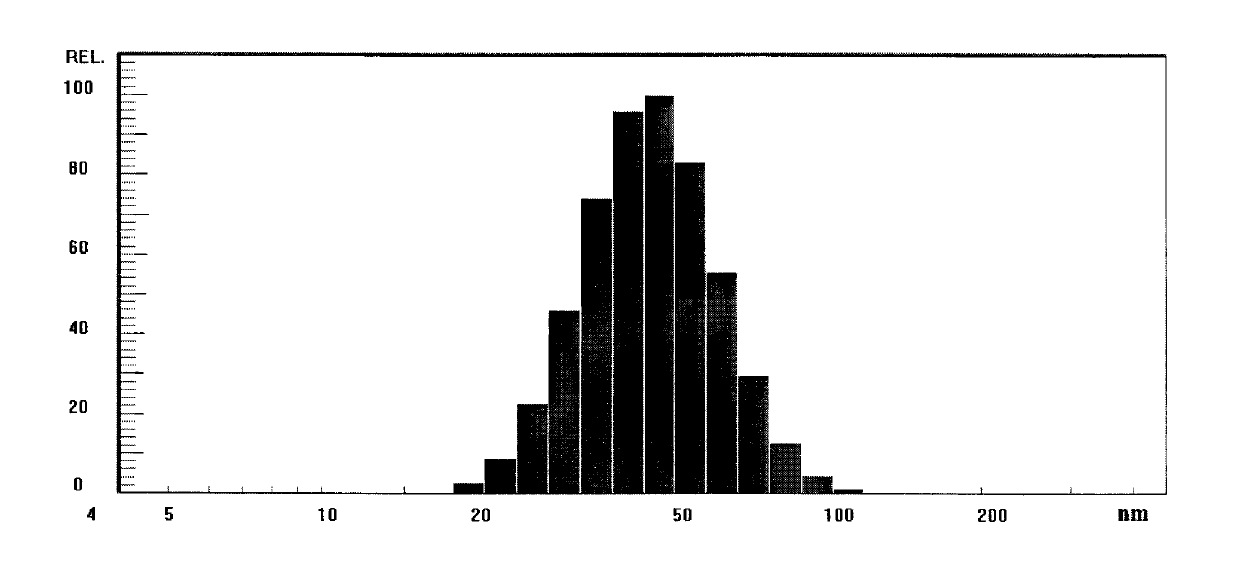
[0037] Nicomp380 was used to measure the particle size, and the docetaxel microemulsion prepared in this example was subjected to particle size detection before freeze-drying, and the results were...
Embodiment 2
[0039] Preparation of lyophilized microemulsion of docetaxel: 6 mg of polyoxyethylene castor oil, 25 mg of soybean oil and 6 mg of polyene phosphatidylcholine were heated and dissolved in 14 mg of isopropanol in a 40°C water bath, and after dissolution, docetaxel was added Add 46 mg of 5% glucose injection (containing 2.3 mg of lactose) slowly, stir while adding, and filter to obtain a transparent microemulsion with opalescence, freeze-dry to remove water, obtain a freeze-dried microemulsion, freeze-dry Remove water to obtain freeze-dried microemulsion.
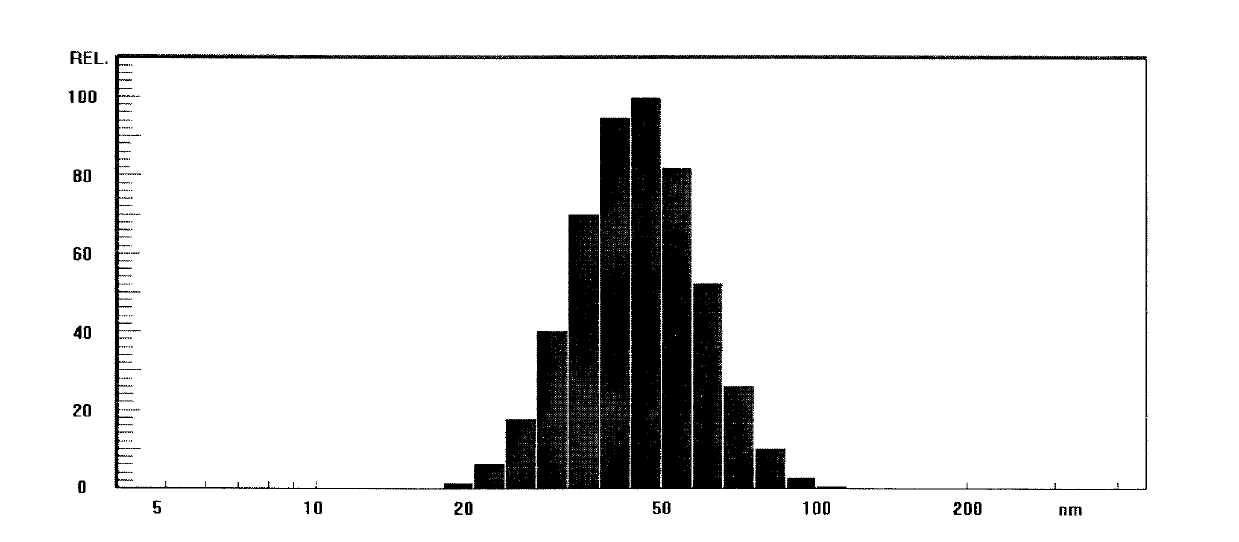
[0040] Preparation of the docetaxel lyophilized microemulsion dilution: the obtained lyophilized microemulsion was redissolved with 0.9% physiological saline to a docetaxel concentration of 0.7 mg / mL. Nicomp380 was used to measure the particle diameter, and the average particle diameter of the diluent was measured to be 41.5nm, and 100% of the particles were below 100nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com