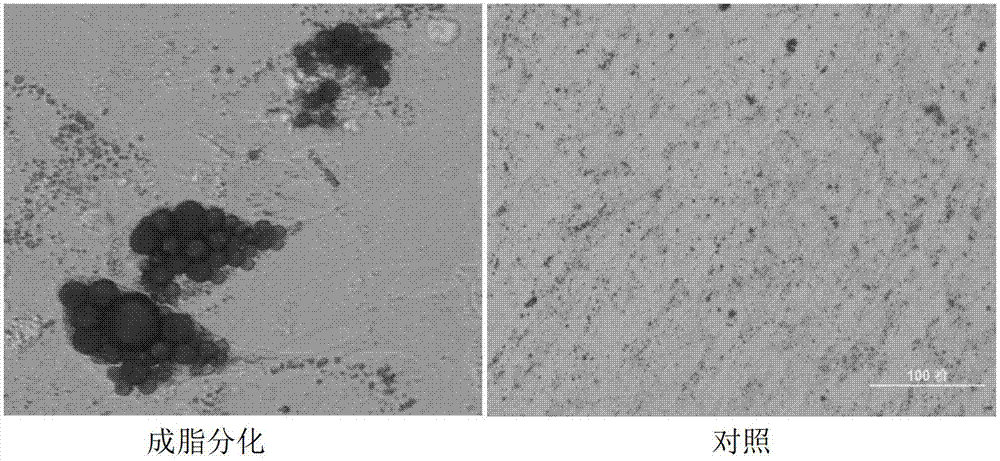
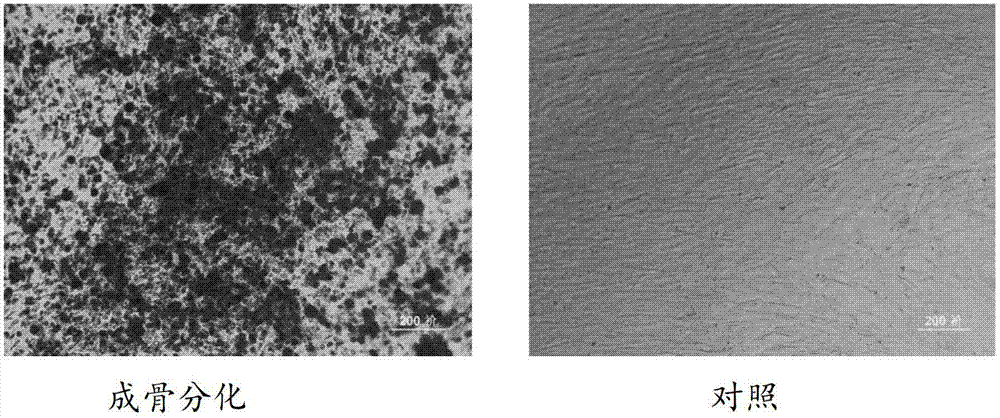
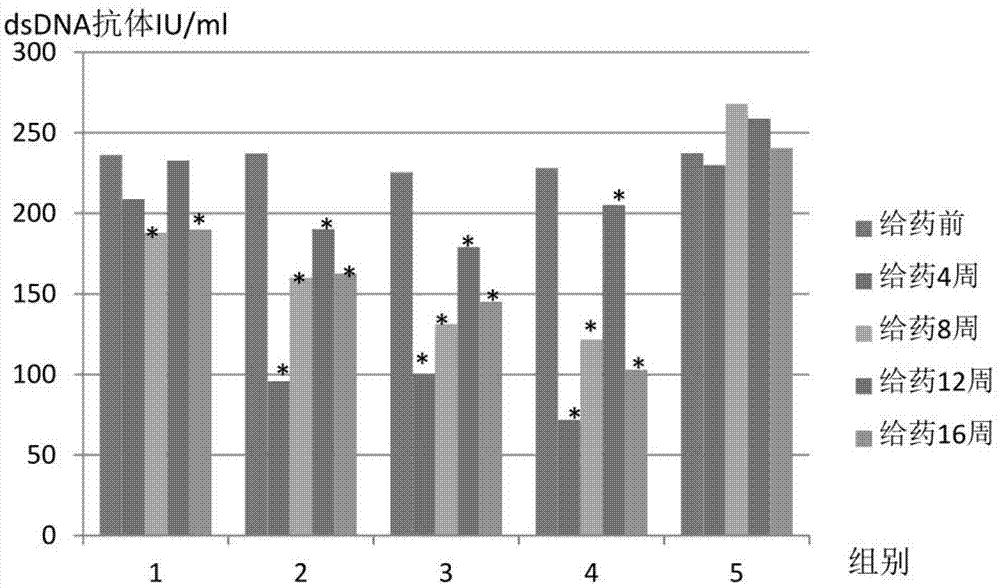
Application of umbilical cord mesenchymal stem cells in preparation of formulation for treating lupus erythematosus
A technology for stem cells and lupus erythematosus, which is applied in the field of stem cells to prepare preparations for treating diseases, can solve problems such as difficulty in obtaining materials, and achieve the effects of high activity, low residue and low preparation cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 1. Stripping of umbilical cord Wharton's jelly
[0070] Immediately after the fetus was delivered, the umbilical cord was ligated and cut according to the obstetrical routine, the umbilical cord was washed with normal saline, and then disinfected with medical alcohol, and the umbilical cord was stored in the umbilical cord preservation solution at a constant temperature of 2-8°C.
[0071] The obtained umbilical cord was rinsed with 0.9% sodium chloride injection, and repeated 2 to 3 times to remove blood stains. The whole umbilical cord was sterilized by immersing it in 75% ethanol. Sodium chloride injection was washed repeatedly to remove residual ethanol. Use sterile surgical scissors to cut the umbilical cord into several sections of about 2 to 5 cm, and remove the congestion and clots in the blood vessels of the small section of the umbilical cord. Remove two arteries and one vein from the umbilical cord. The white connective tissue located between the amniotic m...
Embodiment 2
[0084] 1. Passage and culture of P0 generation cells
[0085] The cell suspension was centrifuged at 300g for 10min. Discard the supernatant, resuspend the cells by gently pipetting with fresh medium, and follow the 5×10 3 piece / cm 2 Density inoculation and passage. Each T175 flask was inoculated with cell suspension, and fresh medium was added. Place in a carbon dioxide constant temperature and humidity incubator to start culturing. Conditions: 37.0±0.5°C, carbon dioxide volume fraction 5.0±0.2%.
[0086] 2. Cell passage
[0087] Cells were cultured for 72±24 hours, and after the cell confluence was observed under an inverted microscope to reach 80%-90%, they could be digested and harvested to obtain P1 generation cells.
[0088] 2.1 Digestion
[0089] Transfer the old culture medium to a sterile container (such as a T175 culture bottle) for later use. Rinse the culture bottle gently with an appropriate amount of 0.9% sodium chloride injection, and discard the washing...
Embodiment 3
[0110] 1. Recovery of cells
[0111] Take out the frozen cells from the liquid nitrogen tank, put them on the recovery rack in the electric heating constant temperature water tank (the water temperature is kept at about 40.0°C), and shake quickly to rewarm. Resuscitation is complete when the cell suspension changes from solid to liquid.
[0112] 1.1 Cell washing:
[0113] Combine the cell suspension into a centrifuge tube. Add pre-cooled 0.9% sodium chloride injection, blow and wash the pipette, constant volume, mix, centrifuge, 300g, 10min.
[0114] 1.2 Sampling counting and washing:
[0115] Discard the washing supernatant, resuspend the cell pellet in an appropriate amount of pre-cooled 0.9% sodium chloride injection, combine it into a centrifuge tube, send the sample to detect the total number of cells and cell viability, centrifuge the cell suspension at 300g, 10min, discard the upper cleared to obtain P3 generation cells.
[0116] 1.3 Passage and cultivation:
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com