Preparation method of crosslinked polystyrene-immobilized benzothiazole catalyst used for formose reaction
A cross-linked polystyrene and benzothiazole technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of non-recyclable and mechanically difficult product separation , uneven mass transfer and other problems, to achieve the effect of easy recycling, easy operation of reaction, and uniform mass transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
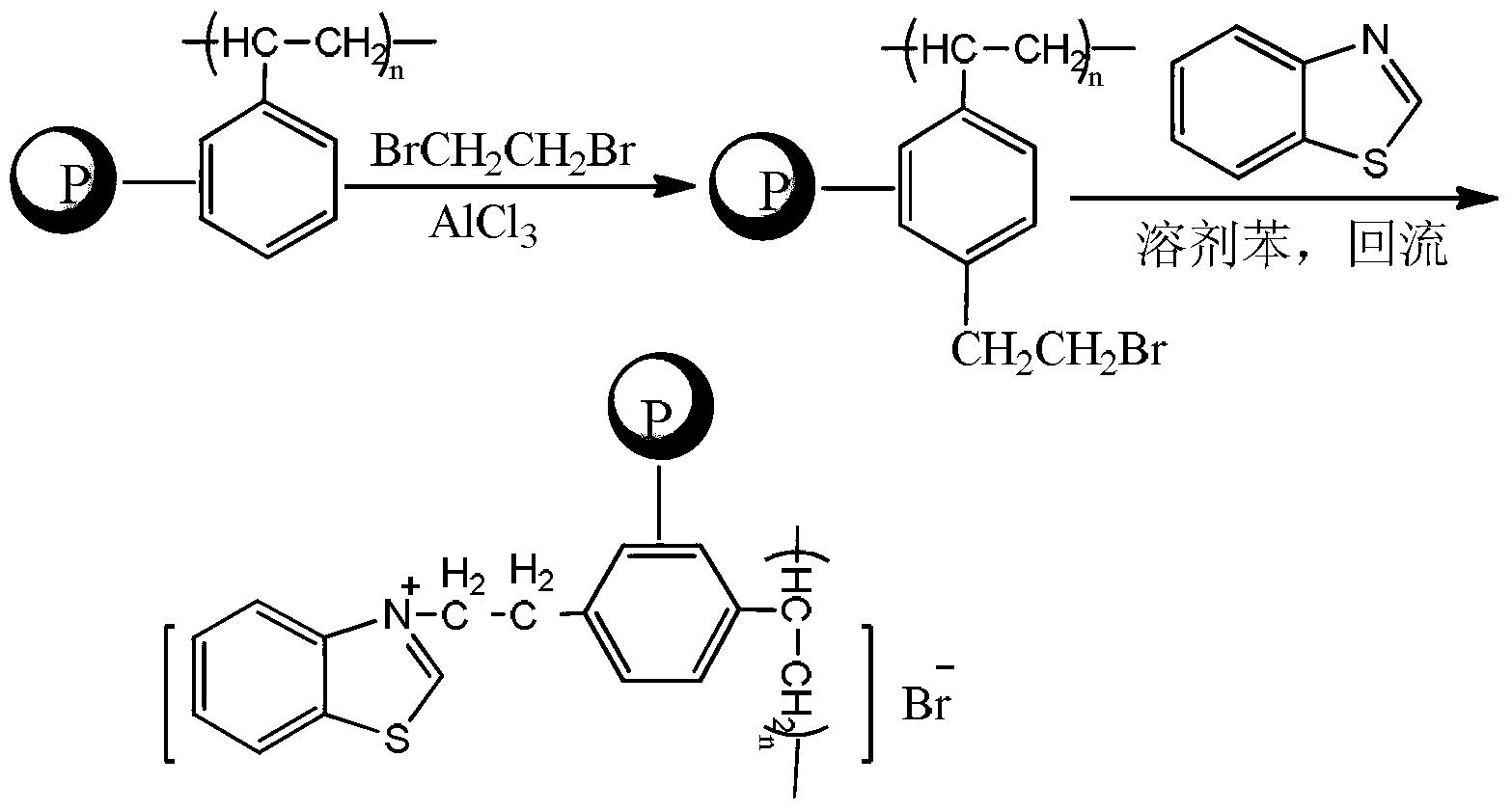
[0031] (1) Preparation of BE-PS:
[0032] Weigh 7g (66.6mmol) of cross-linked polystyrene (cross-linking degree 4%) after long-term low-temperature drying (drying temperature 60°C, drying time 10h), add it to 40ml 1,2-dichloroethane In the three-necked flask, swell at room temperature for 2h, then add 4g of anhydrous AlCl to the three-necked flask 3 , and slowly add 60g of 1,2-dibromoethane dropwise, and stir the reaction at 50°C for 20h, then stop the reaction, cool to room temperature, and then use ethanol, CCl 4 , washed with distilled water, and then dried at 60°C for 10 hours to obtain bromoethylated cross-linked polystyrene (BE-PS). The content of bromine atoms measured by the Volhard method is 17.65% (mass fraction), 2.21mmol / g (the number of moles of bromine atoms per gram of BE-PS).
[0033] (2) Preparation of BETZB-PS:
[0034] Weigh 7g of BE-PS, add it to a three-necked flask containing 30ml of 1,2-dichloroethane, add 10g of benzothiazole, reflux for 24 hours, co...
Embodiment 2
[0038] (1) Preparation of BE-PS:
[0039] Weigh 7g (66.6mmol) of cross-linked polystyrene (cross-linking degree: 2.5%) after long-term low-temperature drying (drying temperature 60°C, drying time 10h), add to 40ml 1,2-dichloroethane In a three-necked flask, swell at room temperature for 2h, and then add 2g of anhydrous AlCl to the three-necked flask 3 , and slowly dropwise added 40g of 1,2-dibromoethane, stirred at 50°C for 15h, stopped the reaction, cooled to room temperature, followed by ethanol, CCl 4 , washed with distilled water, and then dried at 60°C for 10 hours to obtain bromoethylated cross-linked polystyrene (BE-PS). The content of bromine atoms measured by the Volhard method was 11.08% (mass fraction), 1.39mmol / g.
[0040] (2) Preparation of BETZB-PS:
[0041] Weigh 7g of BE-PS, add it into a three-necked flask containing 40ml of 1,2-dichloroethane, add 10g of benzothiazole, reflux for 40h, cool to room temperature after the reaction, and use 1,2-dichloroethane ...
Embodiment 3
[0043] (1) Preparation of BE-PS:
[0044] Weigh 7g (66.6mmol) of cross-linked polystyrene (cross-linking degree: 7%) after long-term low-temperature drying (drying temperature 60°C, drying time 10h), add to 40ml 1,2-dichloroethane In the three-necked flask, swell at room temperature for 2h, then add 4g of anhydrous AlCl to the three-necked flask 3 , and slowly add 40g of 1,2-dibromoethane dropwise, and stir the reaction at 40°C for 20h, then stop the reaction, cool to room temperature, and then use ethanol, CCl 4, washed with distilled water, and then dried at 60°C for 10 hours to obtain bromoethylated cross-linked polystyrene (BE-PS). The content of bromine atoms measured by the Volhard method is 13.93% (mass fraction), 1.74mmol / g (the number of moles of bromine atoms per gram of BE-PS).
[0045] (2) Preparation of BETZB-PS:
[0046] Weigh 7g of BE-PS, add it to a three-necked flask containing 40ml of 1,2-dichloroethane, add 5g of benzothiazole, reflux for 15h, cool to roo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com