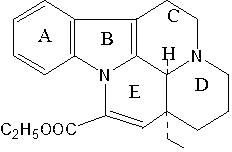
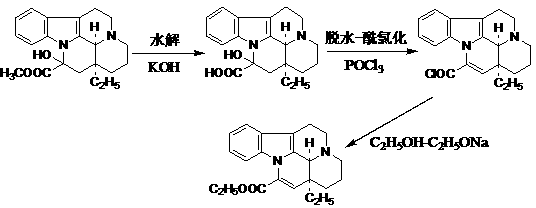
Method for efficiently synthesizing vinpocetine from vincamine
A technology of vinpocetine and vincamine, applied in the direction of organic chemistry and the like, can solve the problems of complicated operation, poor effect, difficult handling, etc., and achieve the effects of simplified reaction steps, high product yield and high acylation efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Add 35 g of potassium hydroxide and 800 ml of absolute ethanol to the three-necked flask, stir at 60°C to dissolve all the potassium hydroxide, then add 71 g of vincamine to the three-necked flask, heat and stir for 4.5 hours, and react on a thin-layer silica gel plate After the reaction is completed, replace the hot water in the water bath with cold water, stir to cool the reaction solution, slowly add glacial acetic acid, and monitor the pH value with pH test paper until the pH is 6. Let it stand for a period of time, filter out the crystals, wash once with an appropriate amount of 300 ml deionized water, rinse with pure water, and dry to obtain 68 g of vinblastine.
Embodiment 2
[0037]Weigh 110 g of vinblastine and put it into a three-necked bottle, measure 1600 ml of dichloroethane, and add 300 ml of phosphorus oxychloride into the bottle, connect the condenser tube and drying tube, adjust the temperature to 70 ° C, and heat and stir for 7 A thin layer of silica gel followed the reaction for hours. After the reaction was completed, the solvent was concentrated under reduced pressure to a minimum amount, the concentrated solution was transferred to a three-necked flask, magnetic stirring was turned on, and 400 ml of absolute ethanol was slowly added dropwise, and then stirred for 30 minutes after the drop was completed. Dilute triethylamine with absolute ethanol to about 60%, and slowly drop it into the three-necked bottle through the constant pressure funnel, and monitor the pH value with pH test paper until the pH value is 8. Continue stirring for 30 minutes after adjusting the pH value. Pour the solid-liquid mixture into a concentrated bottle, con...
Embodiment 3
[0039] Weigh 14 g of sodium hydroxide into a three-necked flask, add 800 ml of absolute ethanol, stir, and heat to 60°C. After the sodium hydroxide was completely dissolved, 36 g of vincamine was weighed and added into a three-neck flask, heated and stirred for 5 hours, and the reaction was followed by TLC. After the reaction is completed, cool to room temperature, slowly add glacial acetic acid, and monitor the pH value with pH test paper until the pH is 6. Let stand for a period of time, filter out the crystals, soak once in an appropriate amount of pure water, rinse with pure water, and dry to obtain 35.3 g of vincine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com