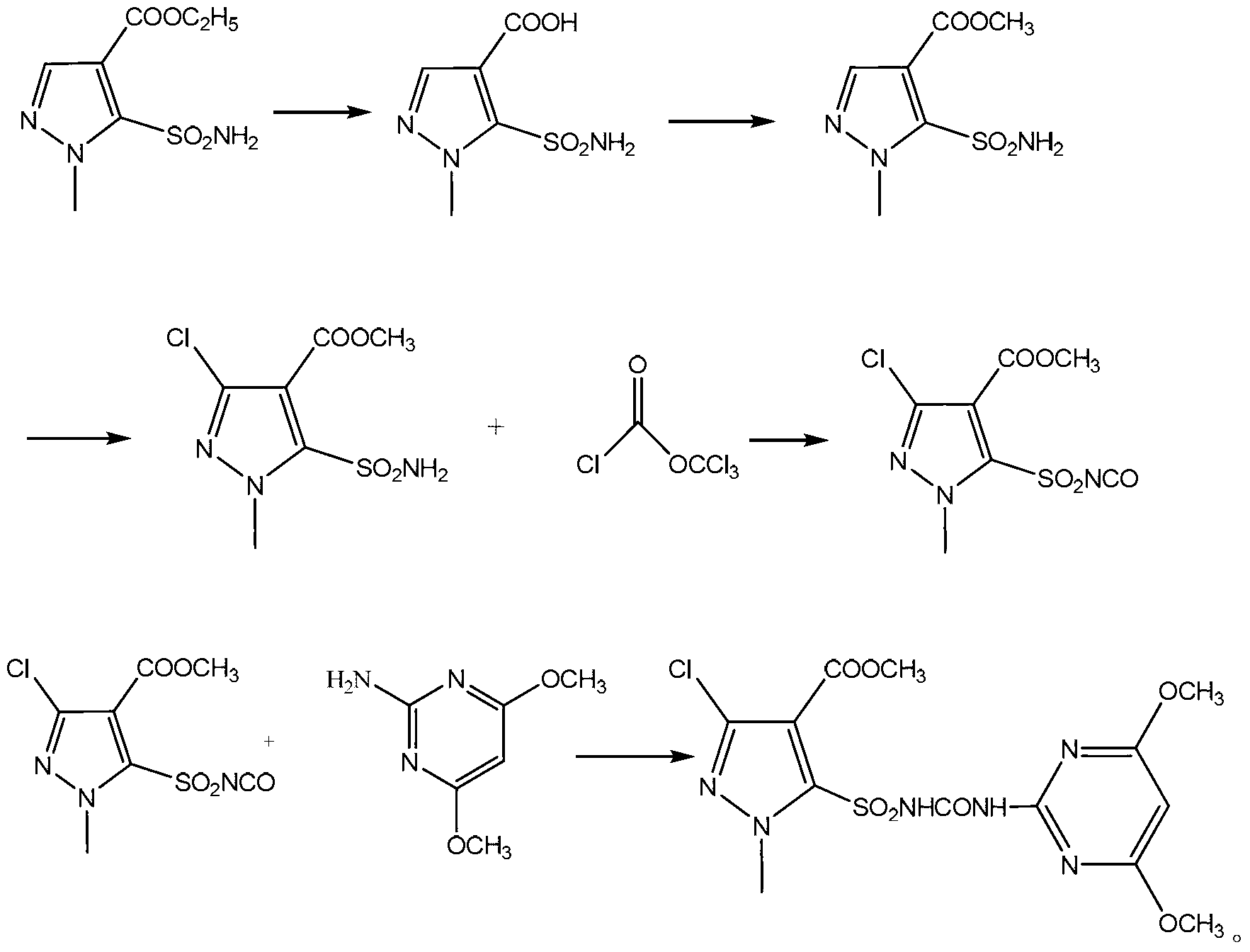
Preparation method of halosulfuron methyl
A technology of chlorpyrifosulfuron and pyrimidine amine, which is applied in the field of herbicide preparation, can solve the problems of difficulty in obtaining, high cost of raw materials, long process route, etc., and achieves the effects of mild reaction conditions, reduced production cost, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
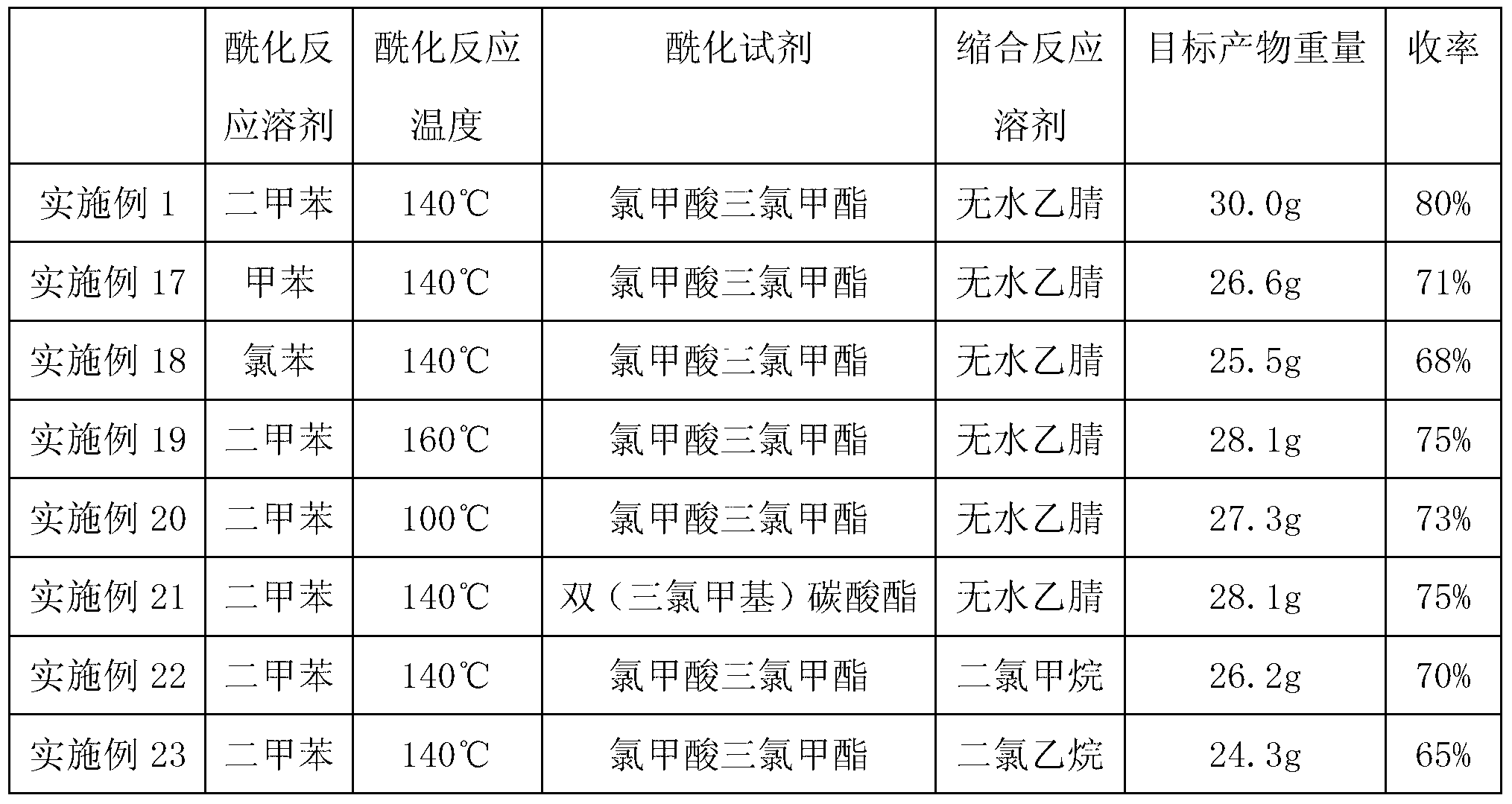
Image
Examples
Embodiment 1)
[0016] The preparation method of the chlorpyrazosulfuron-methyl of the present embodiment is as follows:
[0017] ①Add 24g of ethyl 1-methyl-5-sulfonamido-4-pyrazolecarboxylate (98wt%, 0.1mol) and 150g of 10wt% sodium hydroxide aqueous solution into a 250mL four-neck flask. After the addition, the temperature was raised to 100°C for 5 hours.
[0018] After the reaction, cool to 20°C, add concentrated hydrochloric acid dropwise until the pH of the reaction system is less than 2, a white solid precipitates, cool in an ice-water bath to 5°C, filter with suction, wash the filter cake with water and dry to obtain 20.5g of white powder 1-Methyl-5-sulfonamido-4-pyrazolecarboxylic acid as solid, yield 99%, purity 99% (HPLC).
[0019] ②Add 100mL of methanol and 20.5g of 1-methyl-5-sulfonamido-4-pyrazolecarboxylic acid (99%, 0.099mol) prepared in step ① into a 250mL four-neck flask, stir to dissolve, and cool in an ice-water bath When the temperature is below 10°C, add 24g of thionyl ...
Embodiment 2~ Embodiment 5)
[0027] The rest of each embodiment is the same as Embodiment 1, except for step ①, see Table 1 for details.
[0028] Table 1
[0029]
Sodium hydroxide aqueous solution concentration
Hydrolysis reaction temperature
Product A weight
yield
Example 1
10wt%
100℃
20.5g
99%
Example 2
5wt%
100℃
18.6g
90%
Example 3
30wt%
100℃
19.0g
92%
Example 4
10wt%
40℃
15.5g
75%
Example 5
10wt%
60℃
17.6g
85%
[0030] .
[0031] In Table 1, Product A is 1-methyl-5-sulfonamido-4-pyrazolecarboxylic acid.
[0032] It can be seen from Table 1 that:
[0033] Under other conditions being the same, the yield of 10wt% sodium hydroxide aqueous solution is higher.
[0034] Under other conditions being the same, the yield is higher when the hydrolysis reaction temperature is 100°C.
Embodiment 6~ Embodiment 9)
[0036] The rest of each embodiment is the same as embodiment 1, the difference lies in step ②, see Table 2 for details.
[0037] Table 2
[0038]
Methylation reaction temperature
Product B weight
yield
Example 1
65℃
21.0g
95%
Example 6
65℃
19.9g
90%
Example 7
65℃
17.7g
80%
Example 8
40℃
18.8g
85%
Example 9
Thionyl chloride
100℃
17.6g
80%
[0039] .
[0040] In Table 2, Product B is methyl 1-methyl-5-sulfonamido-4-pyrazolecarboxylate.
[0041] It can be seen from Table 2:
[0042] Under other conditions being the same, using thionyl chloride as the methylation reagent, the yield is higher.
[0043] Under other conditions being the same, the methylation reaction temperature was 65°C and the yield was higher.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com