HPV pseudovirus, kit thereof and method for detecting HPV neutralizing antibodies
A pseudovirus and kit technology, applied in the field of immunology, can solve the problems of long operation cycle, high background value, limited HPV genotype, etc., and achieve the effects of less sample demand, objective result interpretation and standardized methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation of embodiment 1 pseudovirus
[0072] 1 Structural gene expression plasmid and reporter plasmid co-transfected 293FT cells
[0073] at 75cm 2 293FT cells were inoculated into 15ml DMEM complete medium (containing 1% double antibody penicillin streptomycin solution (purchased from Hyclone Company), 1% L-glutamine (purchased from Hyclone Company), 1% non- Essential amino acids (purchased from Hyclone, 10% FBS (purchased from Invitrogen)) at 37°C, 5% CO 2 cultured in an incubator. When the cells grow to a confluence rate of more than 85%, the cell culture medium is aspirated, and 6ml of PBS is added to wash the cells, then the PBS is aspirated, and 3ml of 0.05% trypsin is added (purchased from Hyclone Company, which is pressed by PBS solution) Dilute the 293FT cells at a volume ratio of 1:5) to digest the 293FT cells, resuspend the cells with DMEM complete medium for 5 minutes and count them.
[0074] Press 4×10 5 cells / ml inoculated the cells in 75cm ...
Embodiment 2
[0111] HPV virus neutralizing antibody detection in embodiment 2 serum
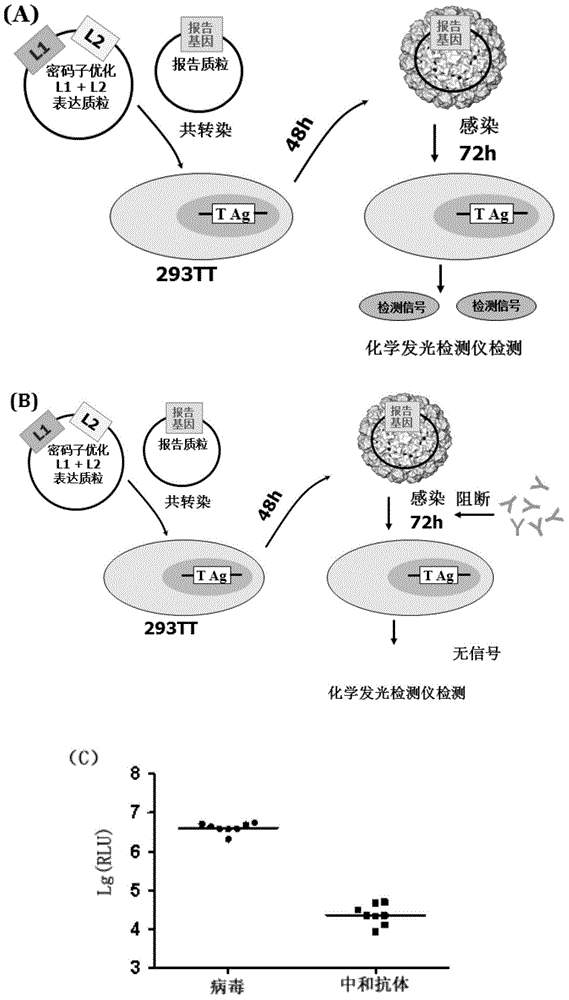
[0112] The World Health Organization (WHO) pointed out in its "Guidelines for the Evaluation of Quality, Safety and Efficacy of HPV VLP Vaccines" that neutralization experiments are the "gold standard" for evaluating whether antibodies induced by HPV vaccines have protective effects.
[0113] In this experiment, after immunizing rabbits with HPV L1 antigens of the above-mentioned 6 genotypes (New Zealand big-eared white, provided by the Laboratory Animal Production and Supply Office of China Food and Drug Control Institute), the same type of pseudovirus and different types of pseudovirus were used to immunize rabbits. The sera after immunization were detected separately, and the steps were as follows:
[0114] (1) Spread 293TT cells on a 96-well culture plate, add 100 μl of 3.0×10 2 293TT cells / μl in complete DMEM medium at 37°C, 5% CO 2 Incubate for 6 hours in the incubator.
[0115] (2) According to ...
Embodiment 3
[0133] Embodiment 3 methodological verification and parameter optimization
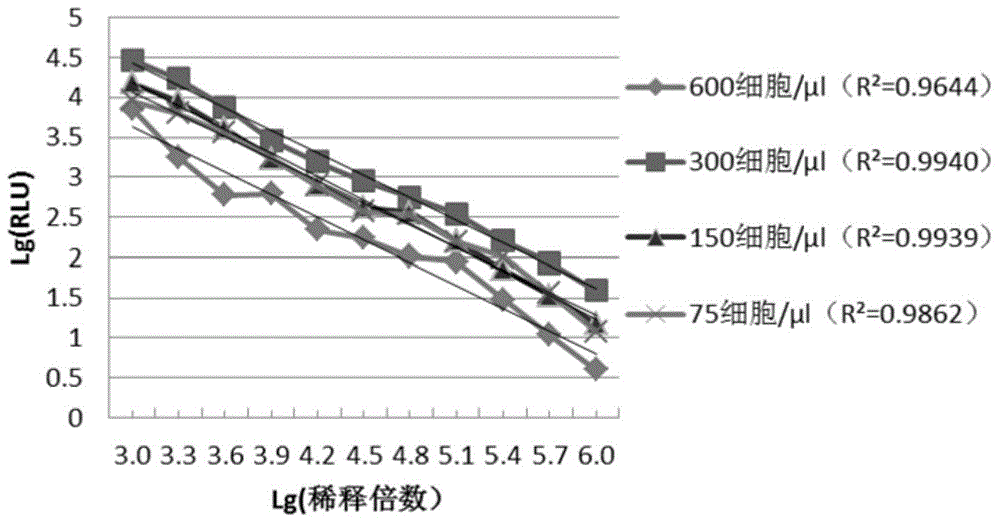
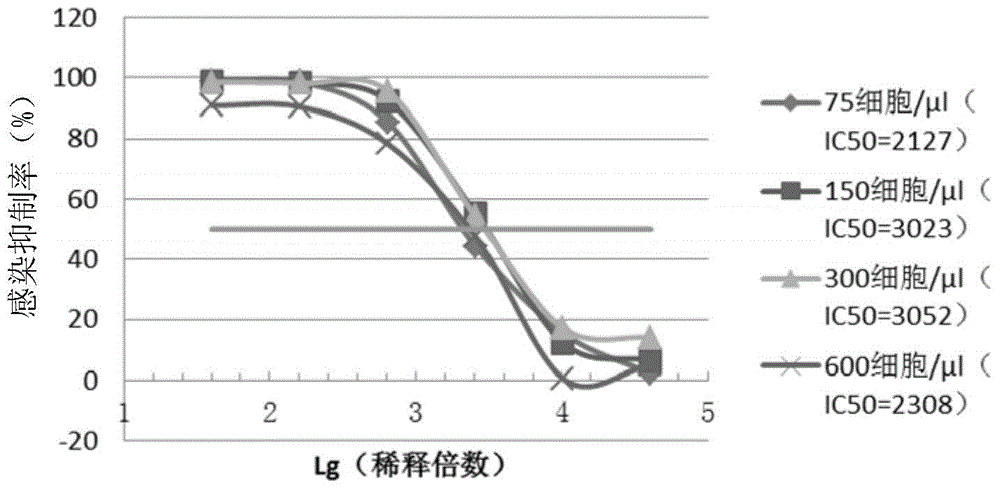
[0134] 1 Selection of cell volume:
[0135] Add 100 μl of cultures of 293TT cells in DMEM complete medium at concentrations of 75 cells / μl, 150 cells / μl, 300 cells / μl and 600 cells / μl to the culture wells of a 96-well cell culture plate . At 37°C, 5% CO 2 Incubate in the incubator for 6 hours, then add an equal volume (100 μl) of pseudovirus dilution, mix well and place at 37°C, 5% CO 2 incubator for a total of 72 hours.
[0136] After co-incubation, transfer 15 μl of the culture supernatant in the cell culture plate to the corresponding wells of the chemiluminescence detection plate, and then add 15 μl of the chemiluminescence detection reagent (same as above) to each well of the chemiluminescence detection plate, and Immediately read in a chemiluminescent detector (same as above).
[0137] (1) Effect of cell volume on virus titer detection:
[0138] The pseudovirus extract obtained in Example ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com