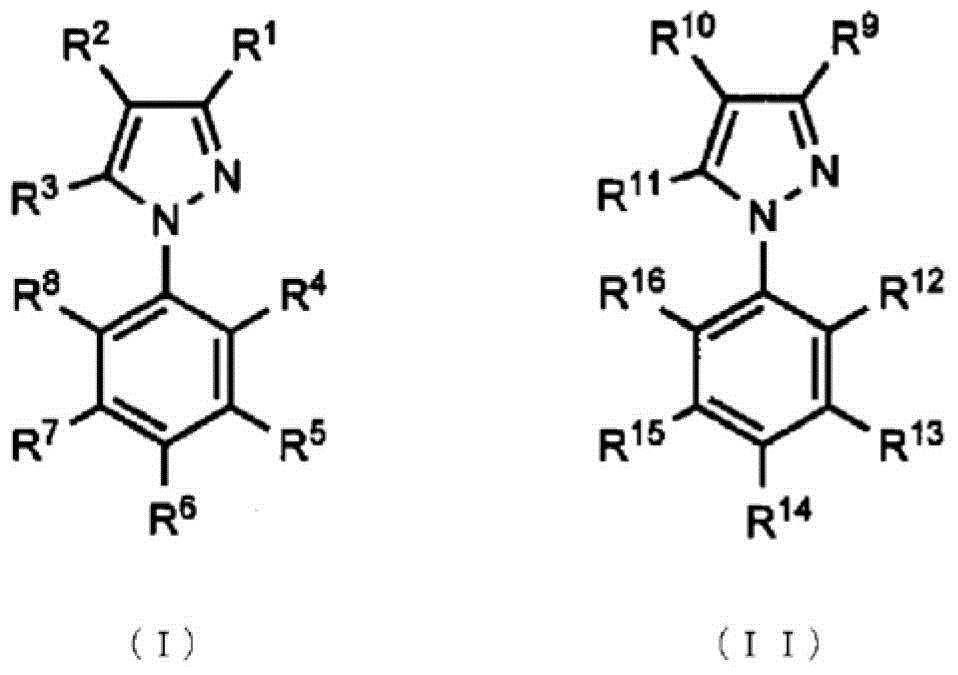
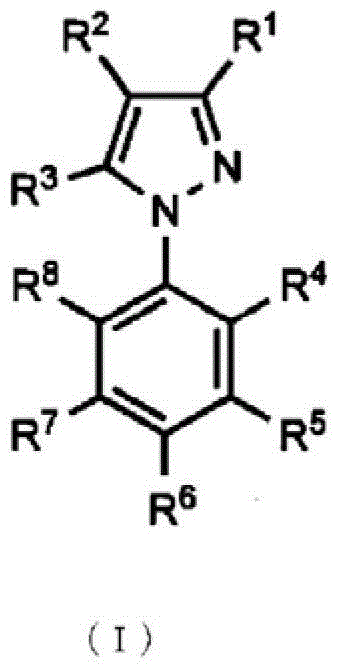
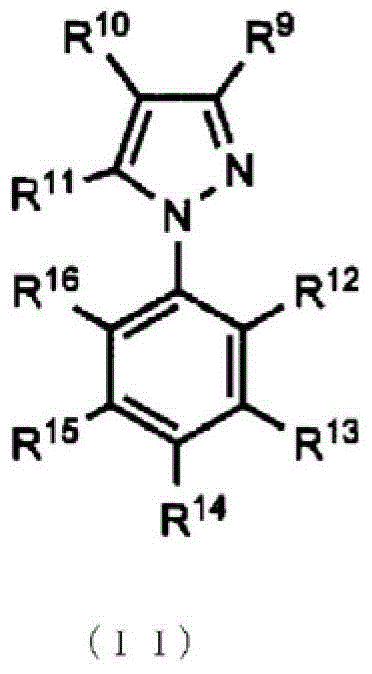
Topical antifungal agent
A technology of alkyl and hydrogen atoms, applied in the field of 2-phenol derivatives or their pharmaceutically acceptable salts, can solve the compounds of 2-(1H-pyrazol-1-yl)phenol skeleton that are not suggested and disclosed Issues such as anti-trichophyton activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] Example 12-(3,5-Dimethyl-1H-pyrazol-1-yl)phenol
[0198] a) 1-(2-methoxyphenyl)-3,5-dimethyl-1H-pyrazole
[0199] Dissolve 3.50 g of 2-methoxyphenylhydrazine hydrochloride in 60 ml of ethanol, add 2.06 ml of acetylacetone, and heat to reflux for 1 hour. 150 ml of water was added to the reaction mixture, neutralized with saturated aqueous sodium carbonate solution, and extracted with 150 ml of ethyl acetate. After the organic layer was dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure and purified by silica gel column chromatography (hexane:ethyl acetate=2:1) to obtain 3.88 g of the title compound.
[0200] 1 H-NMR (CDCl 3 ): δ(ppm)2.08(3H,s),2.29(3H,s),3.78(3H,s),5.95(1H,s),6.98-7.03(2H,m),7.29-7.32(1H,m ),7.34-7.39(1H,m).
[0201] MS(ESI):m / z203(M+H) +
[0202] b) 2-(3,5-Dimethyl-1H-pyrazol-1-yl)phenol
[0203] Dissolve 3.88g of 1-(2-methoxyphenyl)-3,5-dimethyl-1H-pyrazole in 40ml of dichloromethane, add 32ml of 1M b...
Embodiment 2
[0206] Example 22-(3,5-Dimethyl-1H-pyrazol-1-yl)-4-fluorophenol
[0207] a) 2-amino-4-fluorophenol
[0208] 300 mg of 4-fluoro-2-nitrophenol was dissolved in 3 ml of ethanol, 120 mg of 10% palladium / carbon was added, and stirred at room temperature for 1 hour under a hydrogen atmosphere. After filtering the insoluble matter, the solvent was distilled off under reduced pressure to obtain 211 mg of the title compound.
[0209] 1 H-NMR (DMSO-d6): δ (ppm) 4.80 (2H, s), 6.09-6.14 (1H, m), 6.34-6.37 (1H, m), 6.53-6.57 (1H, m), 8.93 (1H ,s).
[0210] MS(FAB):m / z128(M+H) +
[0211] b) 2-(3,5-Dimethyl-1H-pyrazol-1-yl)-4-fluorophenol
[0212] 0.8 ml of 5N-hydrochloric acid was added to 100 mg of 2-amino-4-fluorophenol, and a solution of 65 mg of sodium nitrite dissolved in 0.2 ml of water was added dropwise at 0°C, followed by stirring for 30 minutes. Then, a solution of 249 mg of stannous chloride dissolved in 0.46 ml of 5N-hydrochloric acid was added dropwise at 0°C, stirred at 0...
Embodiment 32
[0215] Example 32-(1H-pyrazol-1-yl)phenol
[0216] a) 1-(2-methoxyphenyl)-1H-pyrazole
[0217] 200 mg of 2-methoxyphenylhydrazine hydrochloride was dissolved in 5 ml of ethanol, 189 μl of malondialdehyde dimethyl acetal was added, and heated to reflux for 2 hours. 50 ml of water was added to the reaction mixture, neutralized with saturated aqueous sodium carbonate solution, and extracted with 60 ml of ethyl acetate. After the organic layer was dried over anhydrous magnesium sulfate, the solvent was distilled off under reduced pressure to obtain 179.4 mg of the title compound.
[0218] 1 H-NMR (CDCl 3 ):δ(ppm)3.87(3H,s),6.42(1H,d,J=2.4Hz),7.02-7.07(2H,m),7.27-7.32(1H,m),7.68-7.72(2H,m ),8.01(1H,d,J=2.4Hz).
[0219] MS(FAB):m / z175(M+H) +
[0220] b) 2-(1H-pyrazol-1-yl)phenol
[0221] In the same manner as in Example 1b), 121 mg of the title compound was obtained from 178 mg of 1-(2-methoxyphenyl)-1H-pyrazole.
[0222] 1 H-NMR (CDCl 3 ):δ(ppm)6.49(1H,d,J=2.4Hz),6.88-6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com