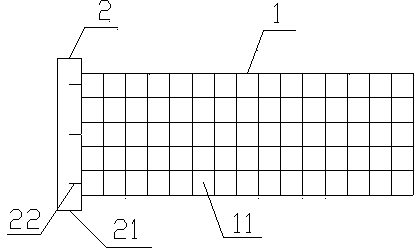
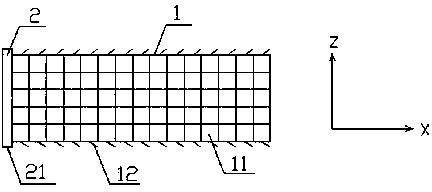
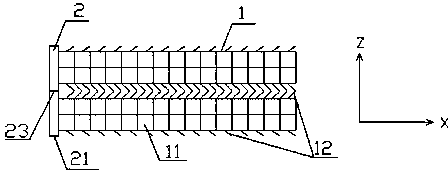
Application of novel slide buckle bio-absorbable stent
A sliding and biological technology, applied in the field of medical devices, can solve problems such as being unsuitable for pediatric vascular stents, hindering lumen reconstruction and expansion, affecting surgical treatment, etc. effect of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Structural Design and Comparison of Bioabsorbable Scaffolds
[0055] According to the current clinical vascular stent structure and the performance of bioabsorbable materials, three structures of stents were designed using PDO materials.
[0056] (1) Self-expanding network pipe support: a stainless steel cylindrical mold is used to obtain the network pipe support. The diameter of the mold is consistent with the diameter of the required bracket. A circle of holes is evenly punched on the circumference of both ends of the mold, and steel needles are inserted. The number of upper and lower steel needles is the same, and they are aligned with each other. The PDO fiber is woven and wound back and forth on the mold. Pay attention to the weaving sequence, you can directly weave a cylinder in which the fibers are interlaced and mutually restricted, and then heat-set (90°C, 4 hours) to keep it in this shape , Weaving density and fiber angle can be adjusted at will.
...
Embodiment 2
[0083] Example 2 In vitro simulated release of four kinds of PDO sliding buckle stents
[0084] (1) Observe the in vitro simulated release of polydioxanone (PDO) snap-in stents
[0085] 1. Materials and methods:
[0086] Materials: Polydioxanone (PDO) buckle-type stents 20*8mm, 10 each, rubber hoses with a diameter of 6 cm, stent delivery systems and pressure pumps.
[0087] method:
[0088] 1. First use the pressure pump to suck the delivery system balloon into negative pressure, then withdraw the outer sheath tube, crimp and wrap the slider brackets on the delivery system balloon respectively, and then push the outer sheath tube forward to the cone to cover stand.
[0089] 2. Insert the stent delivery system into the target site of the artificial blood vessel along the guide wire, expand and release the stent at 12atm*30 seconds.
[0090] 3. Suction the balloon back into negative pressure and withdraw the balloon.
[0091] 2. Observation indicators:
[0092] 1. Vascula...
Embodiment 3
[0133] Example 3 In vitro simulated release of edge-sliding buckle-type stents of five materials
[0134] (1) Observe the simulated release of polydioxanone (PDO) edge-sliding buckle stents in vitro
[0135] 1. Materials and methods:
[0136] Materials: Polydioxanone (PDO) edge-slip buckle-type stents 20*8mm, 10 each, rubber hoses with a diameter of 6 cm, a stent delivery system, and a pressure pump.
[0137] Methods: (see Observation of Polydioxanone (PDO) snap-on stent simulated release in vitro).
[0138] 2. Observation indicators:
[0139] (See Observation of Polydioxanone (PDO) snap-on stent simulated release in vitro).
[0140] 3. Results:
[0141] All the PDO edge-sliding buckle stents can be released under the normal release pressure (10-14atm), and all the stents can be successfully buckled without curling into the lumen; the stent basically maintains the preset lumen diameter, and has a very low Acute elastic recoil (0.3%). It is proved that the stent design is...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com