Synthesis method of benzoxazin ketone compound
The technology of a benzoxazinone and a synthesis method is applied in the field of synthesis of 2-substituted benzoxazinone compounds, which can solve problems such as harsh reaction conditions, equipment corrosion, and high toxicity of reagents, and achieve mild reaction conditions, The effect of low production cost and high synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
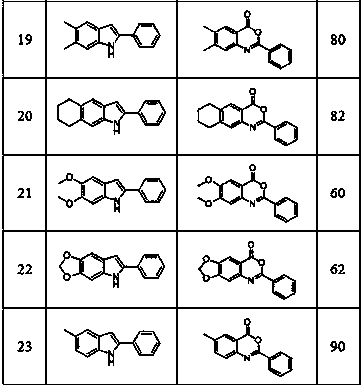
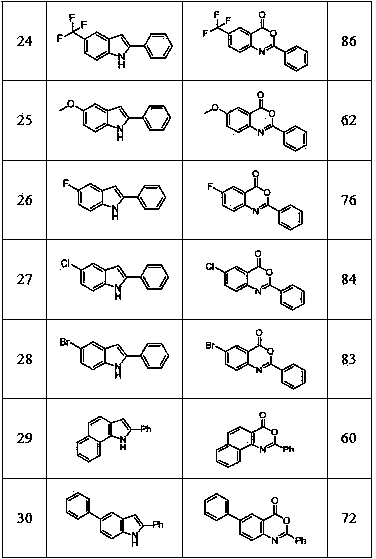
[0015] Embodiment 1: Prepare 2-phenylbenzoxazinone by 2-phenylindole
[0016]
[0017] Into a 50mL round bottom flask, add 2-phenylindole (0.97 g, 0.005 mol), potassium monopersulfate complex salt (9.23g, 0.015 mol) and 20mL of nitromethane, and heat to 90°C in air, The reaction was detected by thin-layer chromatography. After the reaction was completed, it was cooled to room temperature. The reaction mixture was extracted with ethyl acetate and water. The organic phases were combined and spin-dried in vacuum. The crude product was separated by recrystallization or column chromatography to obtain 0.95 g of pure product , the product is a white solid, and the yield is 85%.
[0018] Structural analysis: 1 H NMR (CDCl 3 , 400 MHz): δ 8.31 (d, J = 8.0 Hz, 2 H), 8.24 (d, J = 8.0 Hz, 1 H), 7.85-7.81 (t, J = 7.6 Hz, 1 H), 7.69 (d, J = 8.0 Hz, 1 H), 7.60-7.50 (m, 4 H); 13 C NMR (CDCl 3 , 100 MHz):δ 159.5, 157.0, 146.9, 136.5, 132.6, 130.2, 128.7, 128.5, 128.3, 128.2, 12...
Embodiment 2
[0019] Example 2: Preparation of 5,7-dimethyl-2-phenylbenzoxazinone from 5,7-dimethyl-2-phenylindole
[0020]
[0021] Into a 10 mL round bottom flask, add 5,7-dimethyl-2-phenylindole (44.2 mg, 0.2 mmol), potassium monopersulfate (369.3 mg, 0.6 mol) and 2.0 mL of nitromethane , heated to 100°C in air, and the reaction was detected by thin-layer chromatography until the end of the reaction. The reaction mixture was extracted with water and ethyl acetate, and the organic phase was combined and dried in vacuum. The crude product of the reaction was separated by column chromatography to obtain the pure product 46.5 mg, the product is a light yellow solid, and the yield can reach 92%.
[0022] Structural analysis: 1 H NMR (CDCl3, 400 MHz): δ 8.17 (d, J = 7.2 Hz, 2 H), 7.72 (s, 1 H), 7.44-7.23 (m, 3 H), 2.48 (s, 3 H), 2.30 (s, 3 H). 13 C NMR (CDCl3, 100 MHz): δ 160.1, 154.9, 142.9, 138.5, 137.8, 135.8, 132.1, 130.5, 128.5, 128.0, 125.6, 116.5, 77.3, 77.0, 66.7, 21.9c. / z for...
Embodiment 3
[0023] Example 3: Preparation of 5-chloro-2-phenylbenzoxazinone from 5-chloro-2-phenylindole
[0024]
[0025] To a 10mL round-bottomed flask, add 5-chloro-2-phenylindole (45.4 mg, 0.2 mmol), potassium monopersulfate (369.3 mg, 0.6 mmol) and 2.0 mL of nitromethane in air Heat to 100°C. After the reaction is over, cool to room temperature. Extract the reaction mixture with water and ethyl acetate. Combine the organic phases and spin them dry in vacuum. The crude reaction product is separated by column chromatography to obtain 43.2 mg of pure product. The product is a white solid , yield 84%.
[0026] Structural analysis: 1 H NMR (CDCl 3 , 400 MHz): δ 8.27 (d, J = 7.2 Hz, 2 H), 8.17 (s, 1 H), 7.74 (d, J = 8.0 Hz, 1 H), 7.63-7.50 (m, 4 H). 13 C NMR (CDCl 3 , 100 MHz):δ 158.4, 157.2, 145.4, 136.8, 133.8, 132.8, 129.7, 128.7, 128.7, 128.3, 127.9, 118.0, 77.3, 77.0, 76.7. HRMS Calcd (ESI) m / z 10 h 8 N 2 Na: [M+Na] + 280.0136, found: 280.0126.
[0027] Use different...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com