Ring metal iridium photosensitizers, synthesis thereof and application thereof in hydrogen preparation by photocatalytic reduction of water
A technology of photosensitizer and ring metal, which is applied in the field of chemical energy, can solve the problems of low activity, dual-core iridium photosensitive development has not been reported in the literature, etc., and achieve high activity, good photocatalytic reduction of water hydrogen production performance, and simple post-treatment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
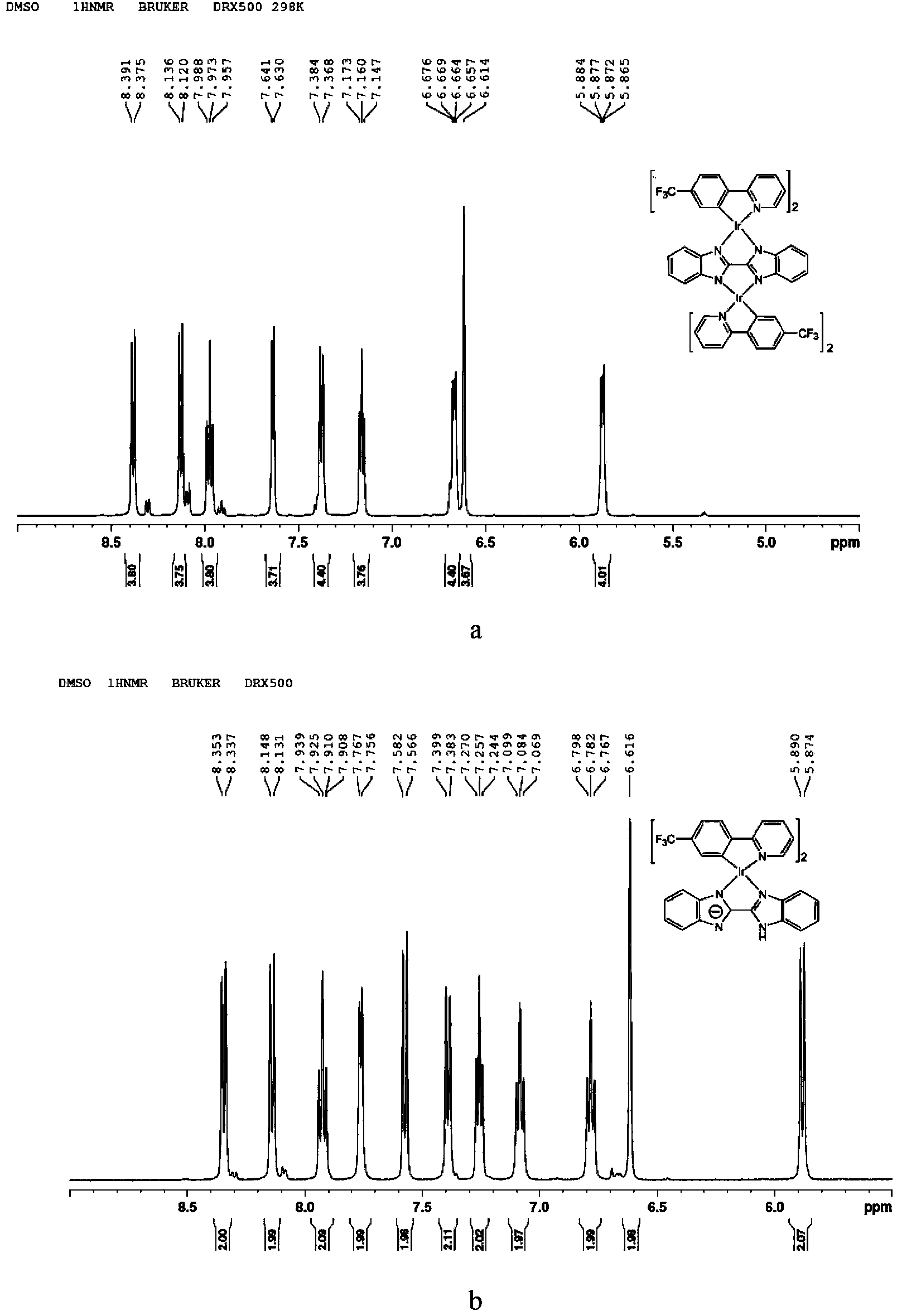
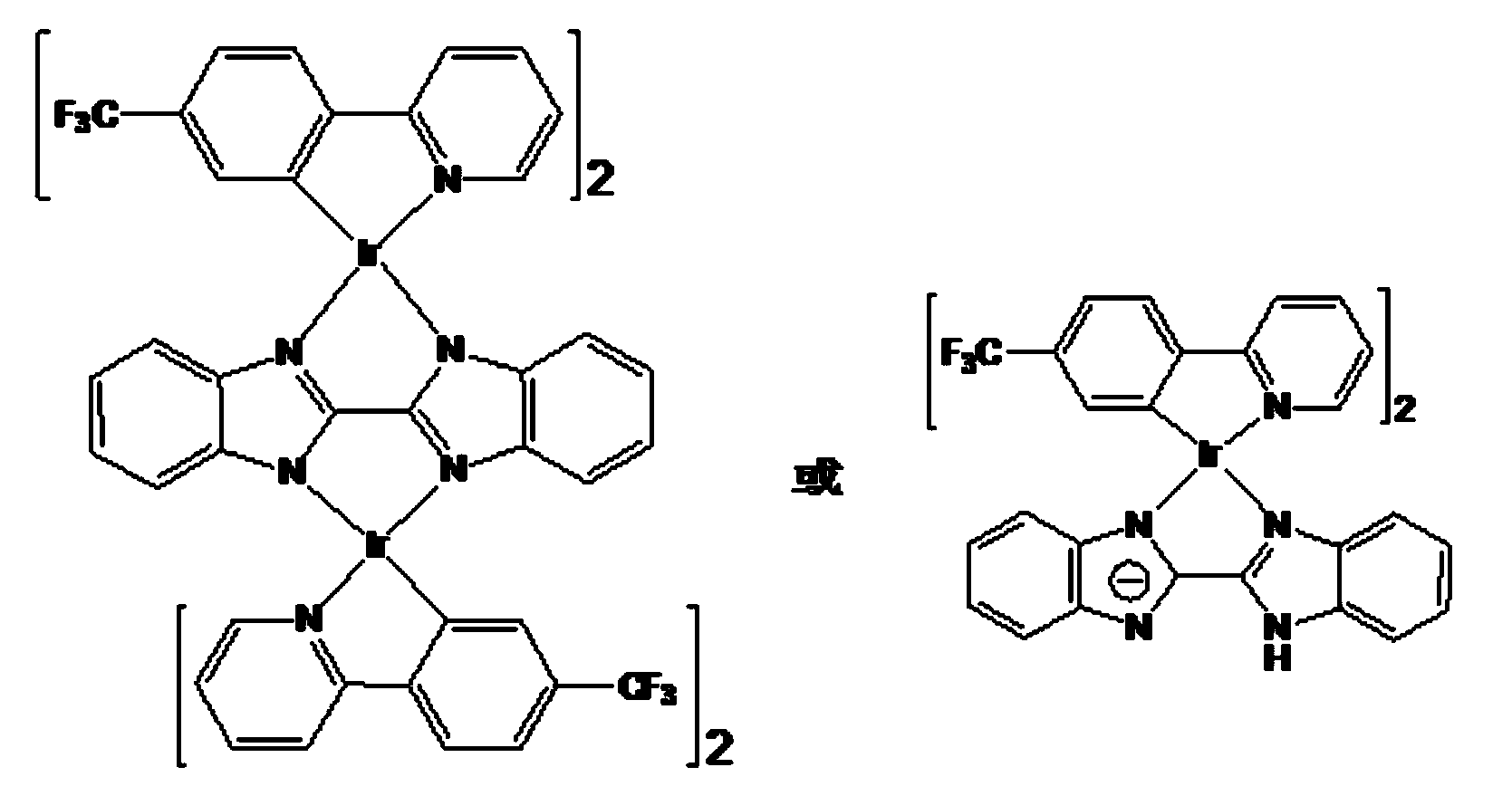
[0027] Example 1: Synthesis of P1 and P2
[0028] Add iridium trichloride hydrate and 4-trifluoromethylphenylpyridine in a ratio of 1:2.2 to 40 ml of ethylene glycol monoethyl ether: water = 3:1, and react at 140 °C for 24 hours under nitrogen protection. After the reaction, the reaction solution was cooled to room temperature, and 100 ml of deionized water was added, at which time a large amount of yellow solids would precipitate. The reaction solution was filtered under reduced pressure to obtain a yellow solid. Dissolve the yellow solid again with dichloromethane, pass through the column with ethyl acetate:n-hexane=1:1, collect the yellow liquid, and obtain pure chlorine bridge dimer [Ir(tfppy)(μ-Cl)] 2 .
[0029] The chlorine-bridged dimer [Ir(tfppy)(μ-Cl)] 2 (300 mg) and bisbenzimidazole (128.7 mg) were added to the mixed solvent CH at a ratio of 1:2.2 2 Cl 2 : CH 3 OH =1:1, add Na 2 CO 3 396 mg, reflux reaction at 70 °C under nitrogen protection for 24 hours. Af...
Embodiment 2
[0033] In a homogeneous photocatalytic system, iridium photosensitizer photocatalytically reduces water to produce hydrogen: add 1 ml 1mol / L iridium photosensitizer P1 or P2, 2.5 ml triethanolamine, 0.33 mM catalyst, 1 g LiCl, 0.75 ml concentrated hydrochloric acid to 100 ml In the solvent (acetone in the solvent: water = 4:1), the air in the reactor was pumped dry, and the hydrogen gas generated by the reaction was quantitatively analyzed by gas chromatography under the irradiation of a 300 W xenon lamp (λ> 420 nm). The amount of hydrogen produced by the photosensitizer under the action of different catalysts is shown in Table 1:
[0034] Table 1
[0035]
[0036] It can be seen from the chart that among the four catalysts, except for CoCl 2 In addition, other catalysts combined with the photosensitizer used have the ability to catalyze the reduction of water to produce hydrogen. Among the many water reduction catalysts used, Rh(dtbpy) 3 3+has the best effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com