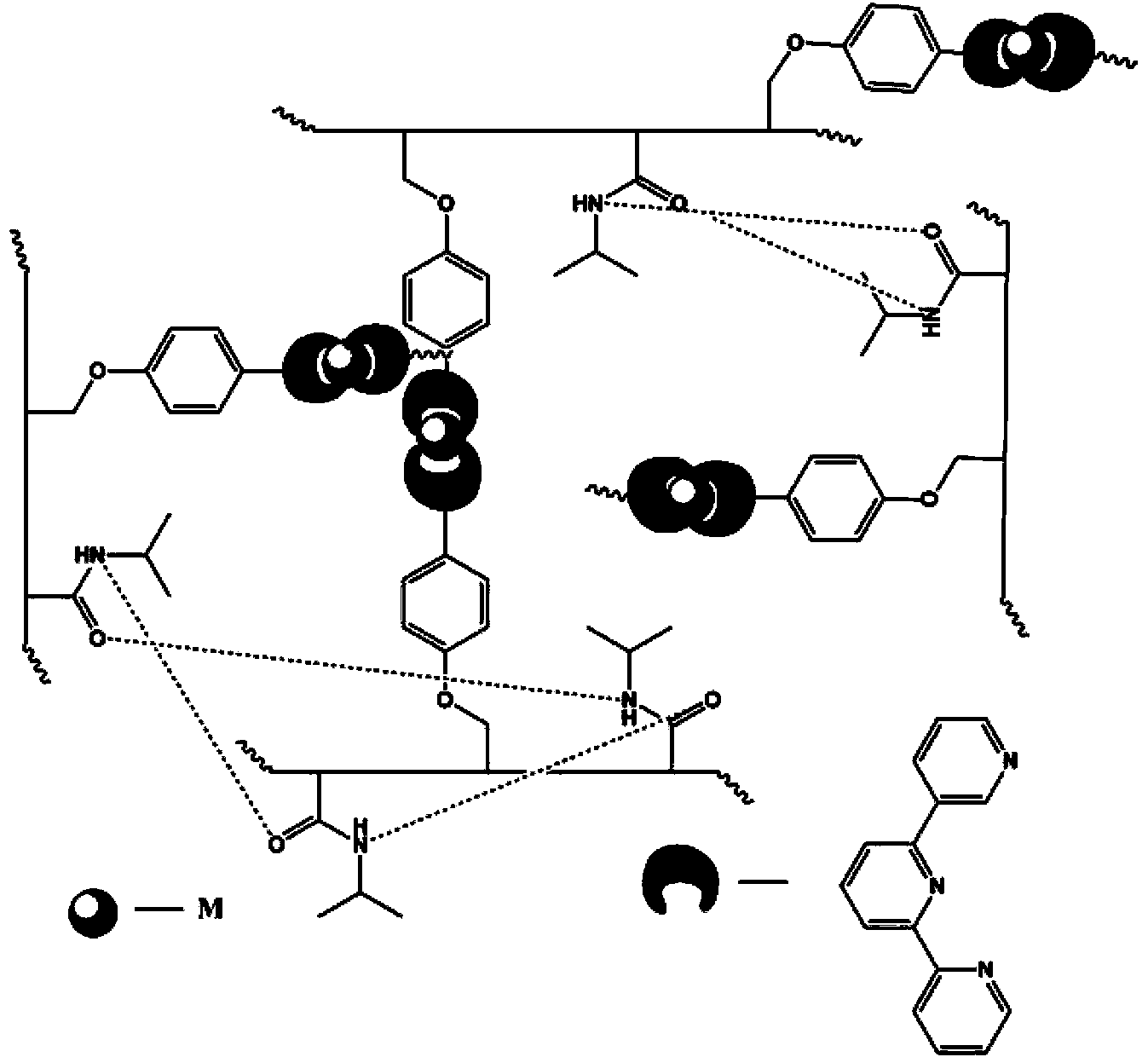
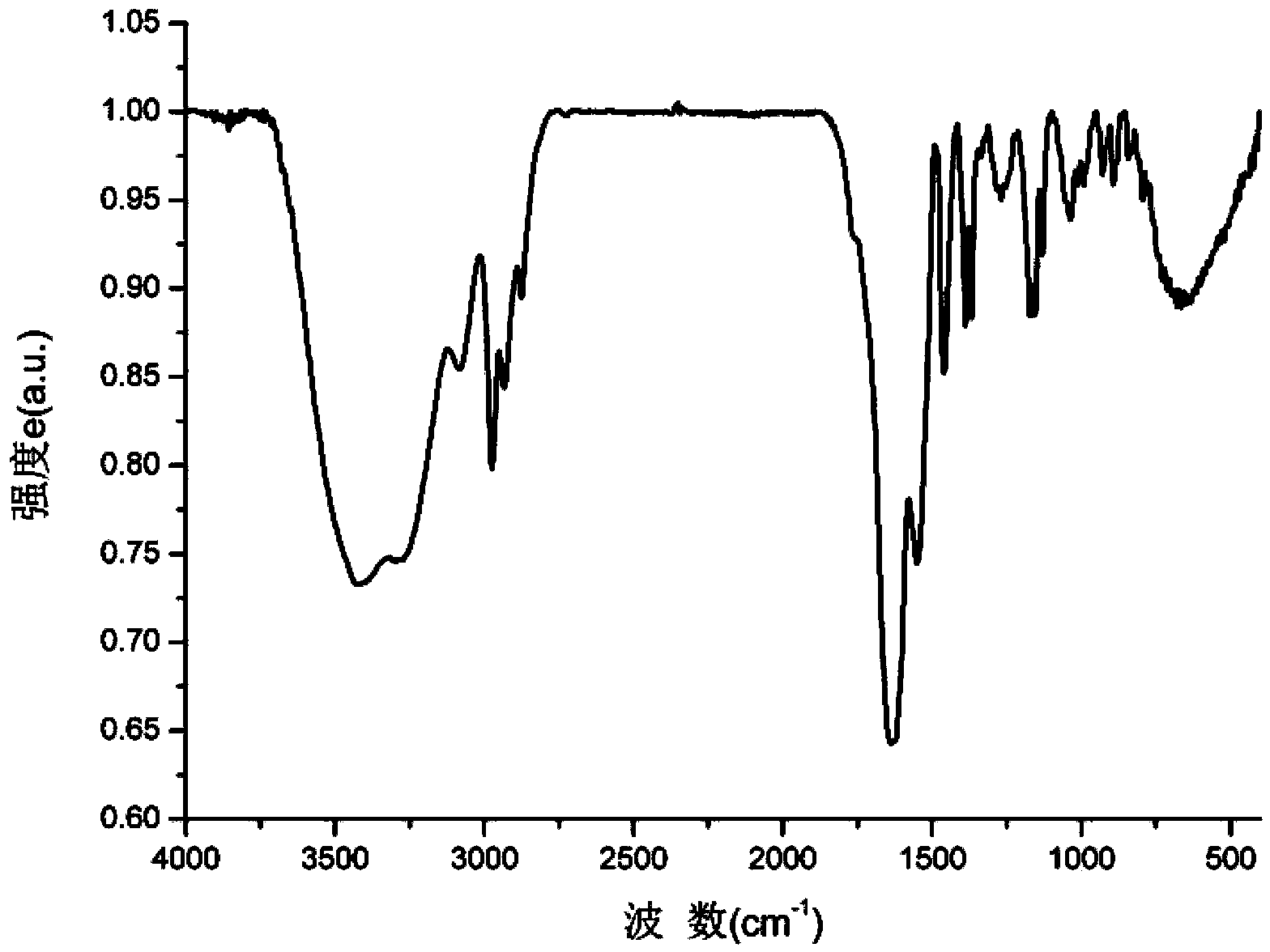
Preparation and application of metal ion directly induced fluorescent supramolecular gel
A supramolecular gel and metal ion technology, applied in the preparation and application of fluorescent supramolecular gel, can solve the problems of industrial complexity, long preparation time, gel instability, etc., and achieve simple preparation process and short gelation time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1. Synthesis of comonomer vinyl-TPY
[0050] (1) Add 10mmol of p-hydroxybenzaldehyde and 10mmol of 4-vinylbenzyl chloride into a 100mL one-necked flask, dissolve completely with 50mL of N,N-dimethylformamide, then add 15mmol of potassium hydroxide, and stir at room temperature for 8 hours. After the reaction, the resulting solution was added to 200 mL of distilled water to obtain a large amount of precipitate, filtered, washed three times with distilled water, and vacuum-dried; the dried product was washed three times with an appropriate amount of n-hexane or cyclohexane, and the obtained solid was dried. Obtain product 4-(4-vinylbenzyloxy)benzaldehyde;
[0051] (2) Add 2.38 grams of 4-(4-vinylbenzyloxy)benzaldehyde (10mmol) and 2.42 grams of 2-acetylpyridine (20mmol) into a 250mL three-necked flask with magnetic stirring, and then add 100mL of anhydrous Ethanol, ultrasound to dissolve the reactant completely, then add 1.55 g of KOH (85%, 23 mmol) and 30 mL ...
Embodiment 2
[0052] Example 2. Preparation of host material terpyridine polymer PNIPAAM-TPY (P1) (n:m=1:100)
[0053] Add 2.5 g of NIPAAm and 0.11 g of vinyl-TPY into the polymerization tube, then add 24.5 mg of initiator AIBN (about 1% of the total mass of the monomer), dissolve with 10 mL of DMF, and if it is difficult to dissolve, sonicate until completely dissolved, then Purge argon 5 times, about 2 minutes each time, seal the tube finally, stir and react at 65°C for 24 hours, dilute the obtained solution with 5mL DMF, then add dropwise to 50mL petroleum ether for reprecipitation, repeat the reprecipitation operation three times , filtered to obtain solid powder, and dried to obtain the target product PNIPAAM-TPY (P1). 1 H NMR (400MHz, CDCl 3 ):δ9.18,8.69,8.32,6.67,4.95,3.96,3.06,2.21,1.78,1.62,1.12. 13 C NMR (100MHz, CDCl 3 ): δ174.5, 170.69, 162.55, 128.553, 115.16, 88.79, 42.28, 41.23, 36.42, 35.13, 22.46.
Embodiment 3
[0054] Example 3. Preparation of host material terpyridine polymer PNIPAAM-TPY (P2) (n:m=5:100)
[0055] Add 2.51 g of NIPAAm and 0.50 g of vinyl-TPY into the polymerization tube, then add 29.7 mg of initiator AIBN (about 1% of the total mass of the monomer), dissolve with 10 mL of DMF, and if it is difficult to dissolve, sonicate until completely dissolved, then Purge argon 5 times, about 2 minutes each time, seal the tube finally, stir and react at 65°C for 24 hours, dilute the obtained solution with 5mL DMF, then add dropwise to 60mL petroleum ether for reprecipitation, repeat the reprecipitation operation three times , filtered to obtain solid powder, and dried to obtain the target product PNIPAAM-TPY (P2). 1 H NMR (400MHz, CDCl 3 ):δ8.70,7.87,7.34,6.92,6.57,5.01,3.95,3.07,2.05,1.76,1.61,1.09. 13 C NMR (100MHz, CDCl 3 ): δ174.43, 170.61, 162.46, 159.55, 156.04, 148.85, 136.76, 128.34, 123.84, 121.21, 118.05, 114.96, 69.67, 42.14, 22.34.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com