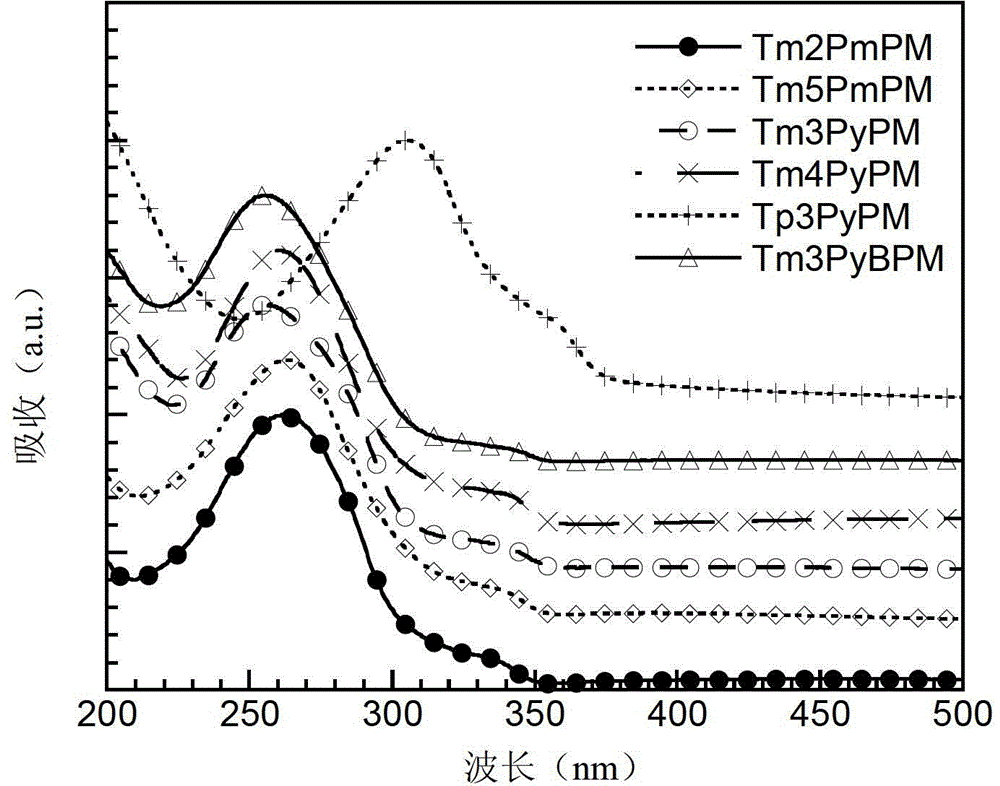
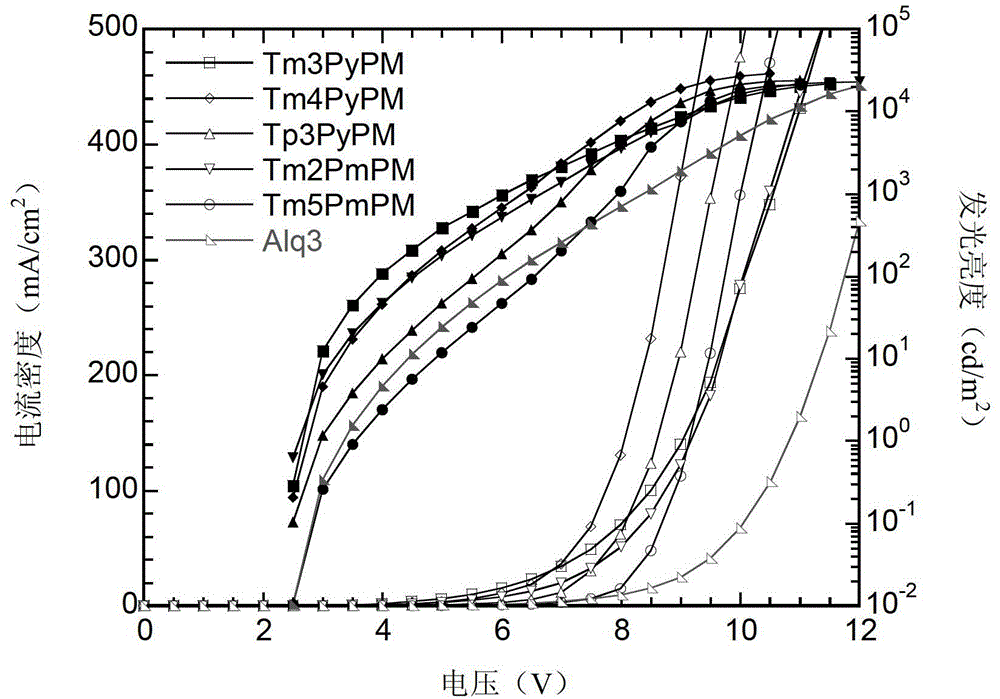
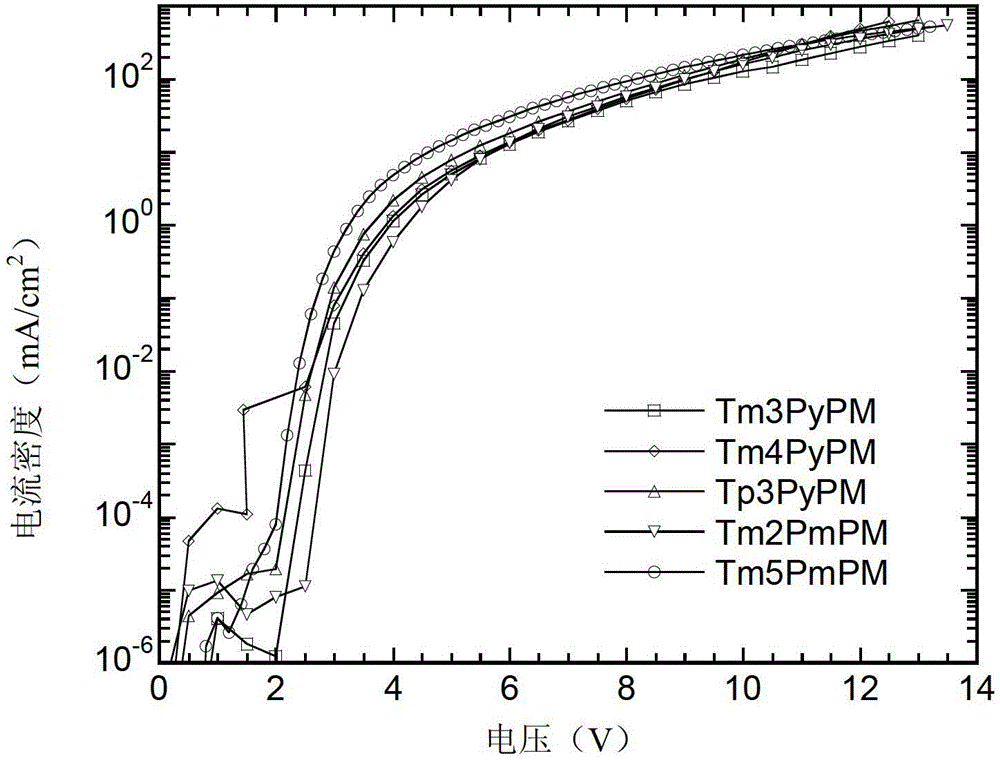
Compound using triphenylpyrimidine as core as well as preparation method and application thereof
A technology of triphenylpyrimidine and compounds, which is applied in the field of organic electroluminescent materials, can solve the problems of increasing electron injection barriers from the cathode to the light-emitting layer, increasing the device driving voltage, and complicating the device structure, so as to suppress energy transfer and improve Effect of device performance, strong electron transport capability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of 2,4,6-tris(4-(3-pyridyl)phenyl)pyrimidine (Tp3PyPM)
[0055] (1) Synthesis of 2,4,6-tris(4-chlorophenyl)pyrimidine
[0056] Under a nitrogen atmosphere, 2,4,6-trichloropyrimidine (3.67g, 20mmol, Aldrich), 4-chlorophenylboronic acid (10.95g, 70mmol, Aldrich), 2M potassium carbonate aqueous solution ( 50ml), bistriphenylphosphine palladium dichloride (0.84g, 1.2mmol, Aldrich) and 1,4-dioxane (120ml, Aldrich), stirred and reacted at 85°C for 8 hours. After the reaction was naturally cooled, the reaction solution was extracted with chloroform, washed three times with saturated brine, and the obtained organic layer was dried over anhydrous magnesium sulfate. Filter with suction, and remove the solvent in the resulting filtrate under reduced pressure. Separation with a chromatographic column, the mobile phase used is chloroform / n-hexane=2:1. After being spin-dried, vacuum-dried to obtain 5.73 g of white powder with a yield of 69.6%.
[0057] 1 H...
Embodiment 2
[0062] Example 2: Preparation of 2,4,6-tris(3'-(3-pyridyl)di-3-phenyl)-pyrimidine (Tm3PyBPM)
[0063] (1) Preparation of pyridylchlorobenzene: Add 3-bromopyridine (4.84g, 30.6mmol, Aldrich), 3-chlorophenylboronic acid (4.95g, 31.6mmol, Aldrich) into a 250ml three-necked flask under nitrogen atmosphere, Tetrakis(triphenylphosphine)palladium (0.70g, 0.61mmol, TCI), 2M potassium carbonate aqueous solution (90ml), toluene (150ml) and ethanol (50ml), stirred and reacted at 85°C for 24h. After the reaction was naturally cooled, the reaction solution was extracted with toluene, washed three times with saturated brine, and the obtained organic layer was dried over anhydrous magnesium sulfate. Filter with suction, and remove the solvent in the resulting filtrate under reduced pressure. Separation with a chromatographic column, the mobile phase used is n-hexane / ethyl acetate=3:1. After spin-dried, vacuum-dried to obtain 5.3 g of a colorless oily product, 3-(3-pyridyl)chlorobenzene, wi...
Embodiment 3
[0075] Example 3: Preparation of 2,4,6-tris(3-(2-pyrimidinyl)phenyl)pyrimidine (Tm2PmPM)
[0076] (1) Synthesis of 2-(3-chlorophenyl)-pyrimidine
[0077] Under a nitrogen atmosphere, 3-chlorophenylboronic acid (5.00g, 32.0mmol), 2-bromopyrimidine (5.08g, 32.0mmol, Aldrich), tetrakis(triphenylphosphine) palladium (0.740 g, 0.64mmol, Aldrich), 2M potassium carbonate aqueous solution (100ml), toluene (150ml) and ethanol (30ml), stirred and reacted under reflux at 85°C for 18 hours. After the reaction was naturally cooled, the reaction solution was extracted with chloroform, washed three times with saturated brine, and the obtained organic layer was dried over anhydrous magnesium sulfate. Filter with suction, and remove the solvent in the resulting filtrate under reduced pressure. Separation with a chromatographic column, the mobile phase used is n-hexane / ethyl acetate=2 / 1. After being spin-dried, vacuum-dried to obtain 5.2 g of white crystals with a yield of 86.3%.
[0078] (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com