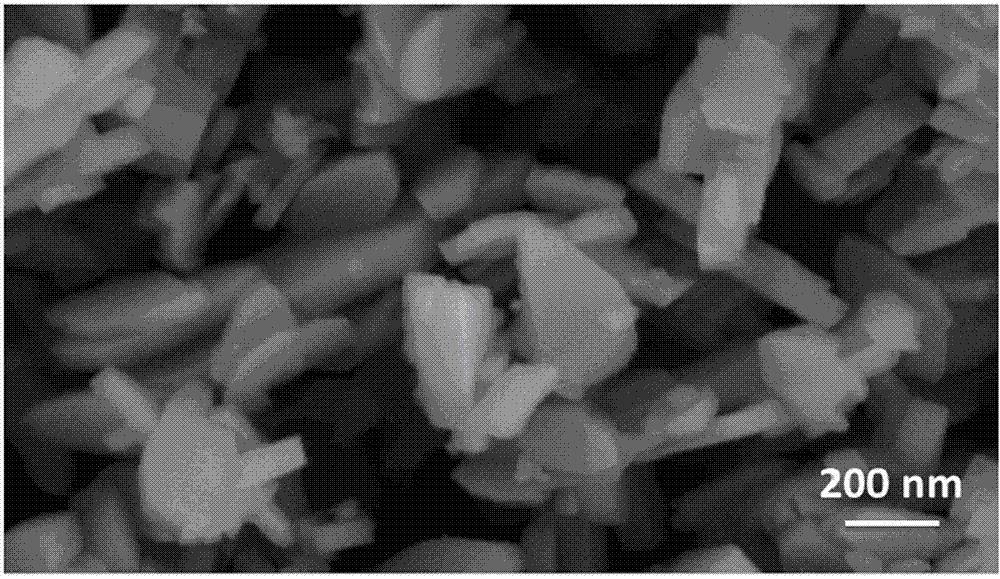
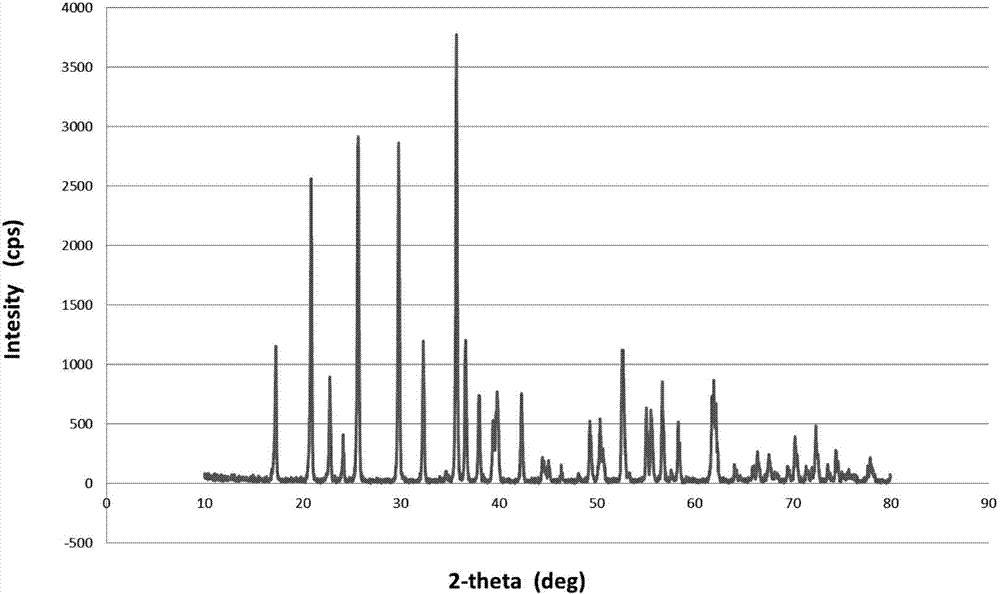
Method for synthesizing nano lithium iron phosphate without water of crystallization through atmospheric water phase
一种水磷酸亚铁锂、无结晶的技术,应用在常压水相合成纳米无结晶水磷酸亚铁锂的制备领域,能够解决不适合大规模的工业化生产、成组电池短板反应、产品批次稳定性差等问题,达到有利于工业化生产、产品性能好、批次稳定的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 4800L of lithium sulfate solution containing 0.2mol / L lithium to a 6300L enamel reaction kettle, start stirring, add 36.9kg of 85% phosphoric acid and raise the temperature to 80°C, then add aqueous sodium hydroxide solution to the reaction kettle to adjust its pH value Adjust to 11, and finally carry out solid-liquid separation and washing to obtain lithium phosphate solid.
[0020] The obtained lithium phosphate was dispersed in water to prepare a lithium phosphate suspension with a concentration of 0.1 mol / L. At the same time, configure 582L ferrous sulfate solution containing 0.5mol / L iron, and adjust its pH value to 1 with sulfuric acid. Add 3000L of the 0.1mol / L lithium phosphate suspension obtained before into a 5000L reactor equipped with a reflux device, start stirring, heat the lithium phosphate suspension to a reflux state, and slowly add the previously configured 582L of ferrous sulfate solution containing 0.5 mol / L of iron, the time used is controlled ...
Embodiment 2
[0024] Add 4800L lithium hydroxide solution containing 0.4mol / L lithium to a 6300L enamel reaction kettle, start stirring, add 73.8% 85% phosphoric acid and raise the temperature to 70°C, then add sodium hydroxide aqueous solution to the reaction kettle to dissolve it The pH value was adjusted to 11, and finally solid-liquid separation and washing were carried out to obtain lithium phosphate solid.
[0025] The obtained lithium phosphate was dispersed in water to prepare a lithium phosphate suspension with a concentration of 0.2 mol / L. Take an appropriate amount of ferrous acetate and ferrous chloride to prepare 582L of a mixed solution of ferrous acetate and ferrous chloride containing 1mol / L of iron, and adjust its pH value to 1 with nitric acid. Add 3000L of the previously prepared 0.2mol / L lithium phosphate suspension into a 5000L reactor equipped with a reflux device, start stirring, and raise the temperature of the lithium phosphate suspension to a reflux state, and slow...
Embodiment 3
[0029] Add 3000L lithium chloride solution containing 4mol / L lithium to a 5000L enamel reaction kettle, start stirring, add 461kg of 85% phosphoric acid and raise the temperature to 20°C, then add sodium hydroxide aqueous solution to the reaction kettle to adjust its pH The value was adjusted to 9, and finally solid-liquid separation and washing were carried out to obtain lithium phosphate solid.
[0030] The obtained lithium phosphate was dispersed in water to prepare a lithium phosphate suspension with a concentration of 1.0 mol / L. Take an appropriate amount of ferrous chloride to configure 1000L of ferrous chloride solution containing 3 mol / L of iron, and adjust its pH value to 3 with hydrochloric acid. Add 3000L of the previously prepared 1.0mol / L lithium phosphate suspension into a 5000L reactor equipped with a reflux device, start stirring, and raise the temperature of the lithium phosphate suspension to a reflux state, and slowly add the previously prepared Configure t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface pH | aaaaa | aaaaa |
| surface pH | aaaaa | aaaaa |
| surface pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com