Calcium gluconate zinc gluconate particulate agent preparation method
A technology of calcium zinc gluconate and calcium gluconate, which is applied in the field of preparation method of calcium zinc gluconate granules, can solve the problems of lowering the quality of medicines, turning yellow or turbid, and low solution stability, and achieves good solubility and improved Taste, the effect of ensuring drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A calcium-zinc gluconate granule, its sugar-containing type contains the following ingredients:
[0026] Calcium gluconate 60 parts
[0027] Zinc gluconate 3 parts
[0028] Lysine hydrochloride 10 parts
[0029] 90 parts of sucrose
[0030] Mannitol 28 parts
[0031] 2 parts citric acid
[0032] Sucralose 0.8 parts
[0033] Peach essence 0.2 parts
[0034] Povidone K30 0.3 parts
[0035] The preparation method is as follows: mixing the raw and auxiliary materials evenly, adding purified water to granulate, drying at 60°C, sizing the granules with a 24-mesh sieve, and packing them into composite film bags.
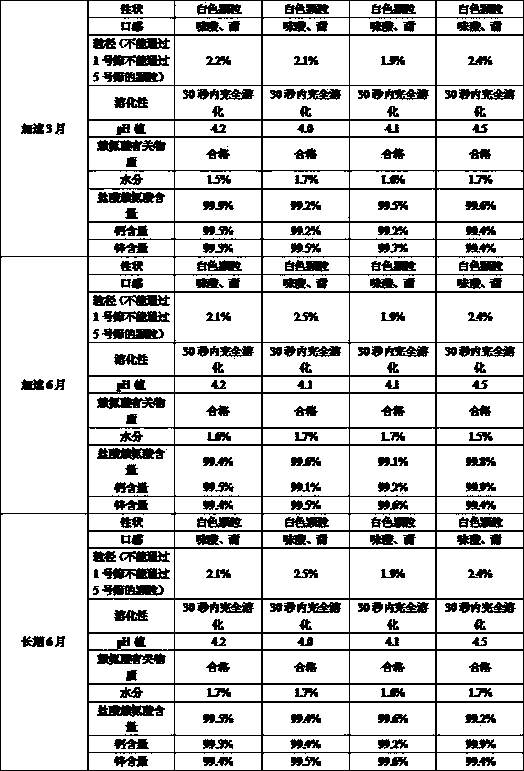
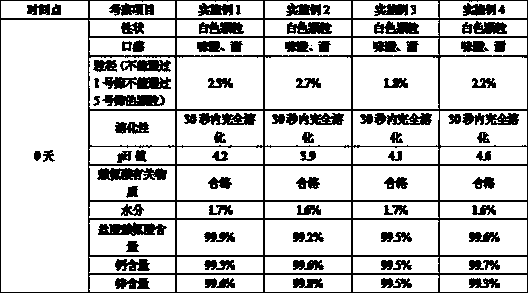
[0036] The calcium zinc gluconate granule preparation of the present invention has a pH value of 4.2.
Embodiment 2
[0038] A calcium-zinc gluconate granule, its sugar-containing type has the following ingredients:
[0039] Calcium gluconate 60 parts
[0040] Zinc gluconate 3 parts
[0041] Lysine hydrochloride 10 parts
[0042] Lactose 40 parts
[0043] Maltose 20 parts
[0044] Mannitol 28 parts
[0045] starch 3 parts
[0046] 4 parts citric acid
[0047] Sodium citrate 2 parts
[0048] Acesulfame K 0.2 parts
[0049] AK sugar 0.2 parts
[0050] Peach essence 0.2 parts
[0051] Sweet orange essence 0.1 parts
[0052] Povidone K30 0.3 parts
[0053] The preparation method is as follows: mixing the raw and auxiliary materials evenly, adding purified water to granulate, drying at 60°C, sizing the granules with a 24-mesh sieve, and packing them into composite film bags.
[0054] The pH value of the calcium zinc gluconate granule preparation of the present invention is 3.9.
Embodiment 3
[0056] A calcium zinc gluconate granule, its sugar-free type has the following ingredients:
[0057] Calcium gluconate 60 parts
[0058] Zinc gluconate 3 parts
[0059] Lysine hydrochloride 10 parts
[0060] Mannitol 70 parts
[0061] Starch 30 parts
[0062] Dextrin 25 parts
[0063] Lactic acid 6 parts
[0064] Stevioside 2 parts
[0065] Peach essence 0.2 parts
[0066] Sweet orange essence 0.1 parts
[0067] Povidone K30 0.3 parts
[0068] The preparation method is as follows: mixing the raw and auxiliary materials evenly, adding purified water to granulate, drying at 60°C, sizing the granules with a 24-mesh sieve, and packing them into composite film bags.
[0069] The calcium zinc gluconate granule preparation of the present invention has a pH value of 4.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com