Chiral aluminum compound and preparation method thereof and preparation method of polylactic acid
A technology of aluminum compound and polylactic acid, which is applied in the field of preparation of polylactic acid, chiral aluminum compound and its preparation, can solve the problems of low selectivity and activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
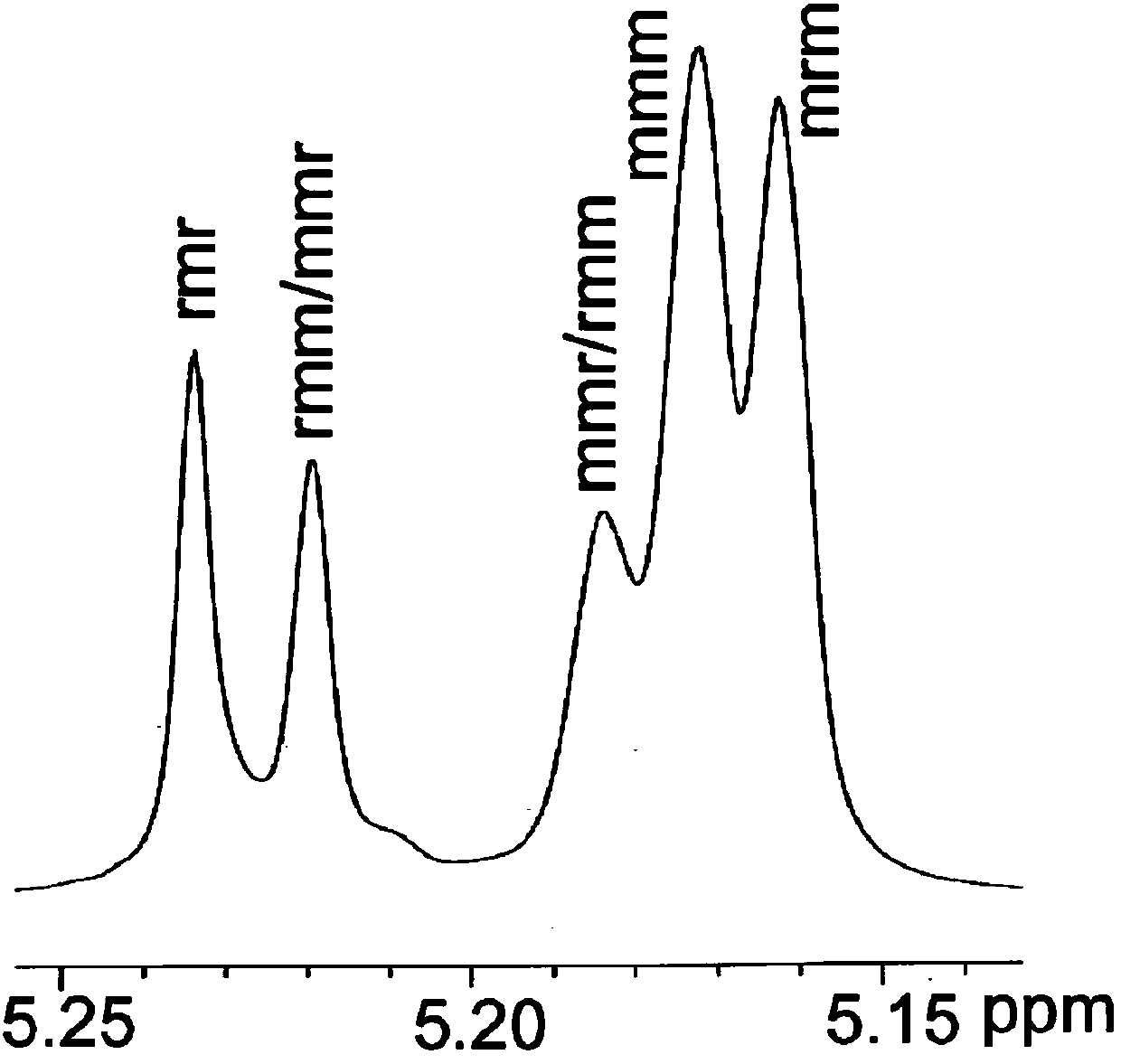
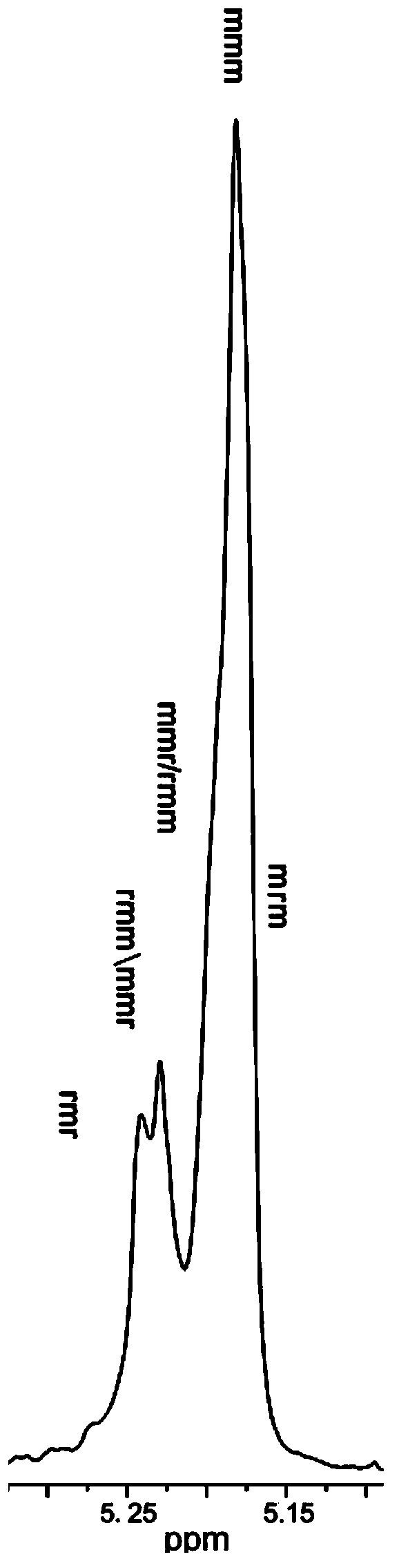
Image
Examples
preparation example Construction
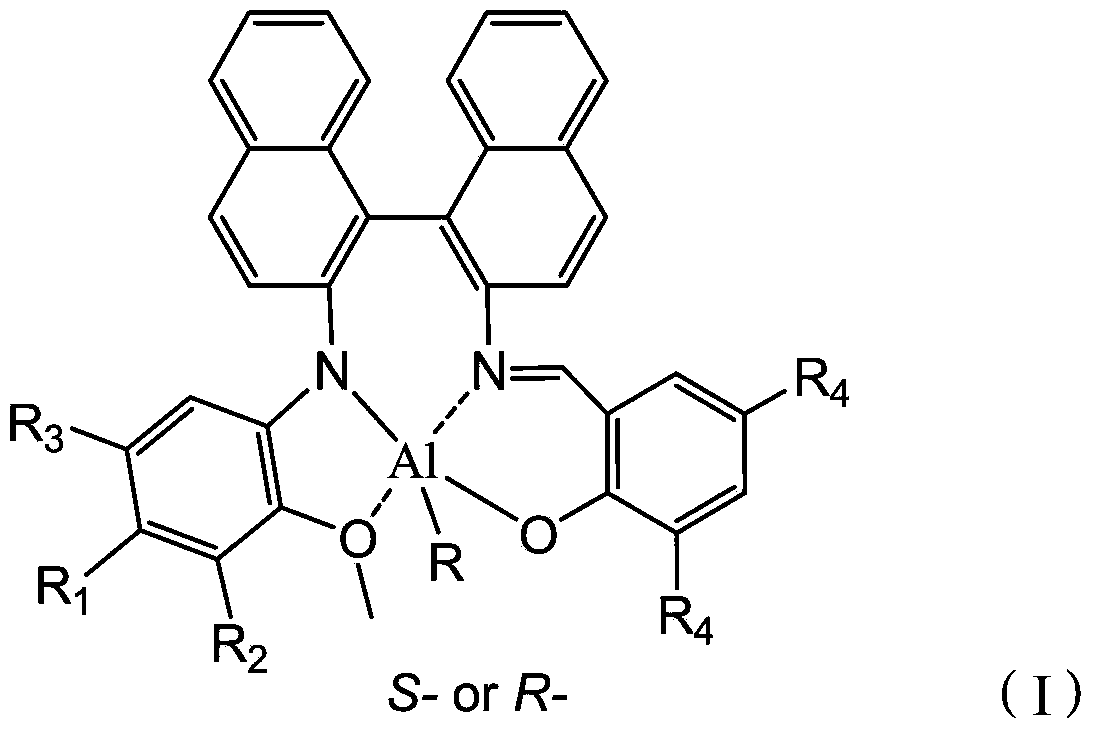
[0049] Optionally, when R is -CH 3 or -CH 2 CH 3 When, the present invention also provides a preparation method of a chiral aluminum compound, comprising the following steps: combining the chiral ligand with the structure of formula (II) with Al(R′) 3 React in a solvent to obtain a chiral aluminum compound with the structure of formula (III). Wherein, the solvent is an organic solvent well known to those skilled in the art, preferably tetrahydrofuran or toluene.
[0050] R 1 , R 2 , R 3 and R 4 The choice of influences the choice of solvent, when R 1 , R 2 , R 3 and R 4 independently selected from -H, -F, -Cl, -Br or -NO 2 When, the reaction solvent is preferably tetrahydrofuran, when R 1 , R 2 , R 3 and R 4 independently selected from -CH 3 、-CH 2 CH 3 、-CH(CH 3 ) 2 、-C(CH 3 ) 3 When, the reaction solvent is preferably toluene.
[0051]
[0052]
[0053] R 1 , R 2 , R 3 and R 4 independently selected from -H, -CH 3 、-CH 2 CH 3 、-CH(CH 3 ) ...
Embodiment 1
[0074] Embodiment 1 structural formula is the synthesis of the ligand IIa of II
[0075] IIa: R1=R2=R3=R4=-H
[0076] Under nitrogen atmosphere, dissolve 0.09g of palladium acetate and 0.50g of binaphthyl diphenylphosphine in 50mL of toluene, slowly add 1.50g of 2-bromoanisole and 2.27g of S-type or R-type 2,2'-diamino-1 , 1'-binaphthyl, stirred at room temperature for 5 minutes, added 1.152g of sodium tert-butoxide, stirred at room temperature for 10 minutes, then placed in a 70°C oil bath and stirred for 6 hours, cooled to room temperature, added 60mL of ether, washed with saline, and separated Afterwards, sodium carbonate was dried, concentrated, and the obtained crude product was separated by column chromatography, and the eluent was hexane:ethyl acetate (volume ratio of 10:1), which included 2% triethylamine, and the obtained product was colorless Solid VIa;
[0077] Dissolve VIa (0.390g, 1.00mmol) and salicylaldehyde (0.122g, 1.00mmol) in 10ml of ethanol, heat to reflu...
Embodiment 2
[0084] Embodiment 2 structural formula is the synthesis of the ligand IIb of II
[0085] IIbR1=-F, R2=R3=R4=-H,
[0086] Under a nitrogen atmosphere, dissolve 0.090 g of palladium acetate and 0.50 g of binaphthyl diphenylphosphine in 50 mL of toluene, slowly add 1.64 g of 2-bromo-5-fluoroanisole and 2.27 g of S-type or R-type 2,2'- Diamino-1,1'-binaphthyl, stirred at room temperature for 5 minutes, then added 1.15g of sodium tert-butoxide, stirred at room temperature for 10 minutes, then placed in a 70°C oil bath for 6 hours, cooled to room temperature, added 60mL of ether, washed with saline After washing and separating, dry with sodium carbonate and concentrate, the obtained crude product is separated by column chromatography, the eluent is hexane:ethyl acetate (volume ratio is 10:1), which includes 2% triethylamine, and finally obtains The product is a colorless solid VIb;
[0087] Dissolve VIb (0.408g, 1.00mmol) and salicylaldehyde (0.122g, 1.00mmol) in 10ml of ethanol, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com