Application of andrographolide derivatives in preparing medicaments for preventing and treating neurodegenerative diseases
A technology of andrographolide and neurodegeneration, which is applied in the field of application of andrographolide derivatives in the preparation of drugs for the prevention and treatment of neurodegenerative diseases, can solve the problems such as the unclear pathogenesis of neurodegenerative diseases, and achieve reduction Effects of oxidative stress injury, reduction of inflammatory response, and inhibition of anti-neuroinflammatory response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
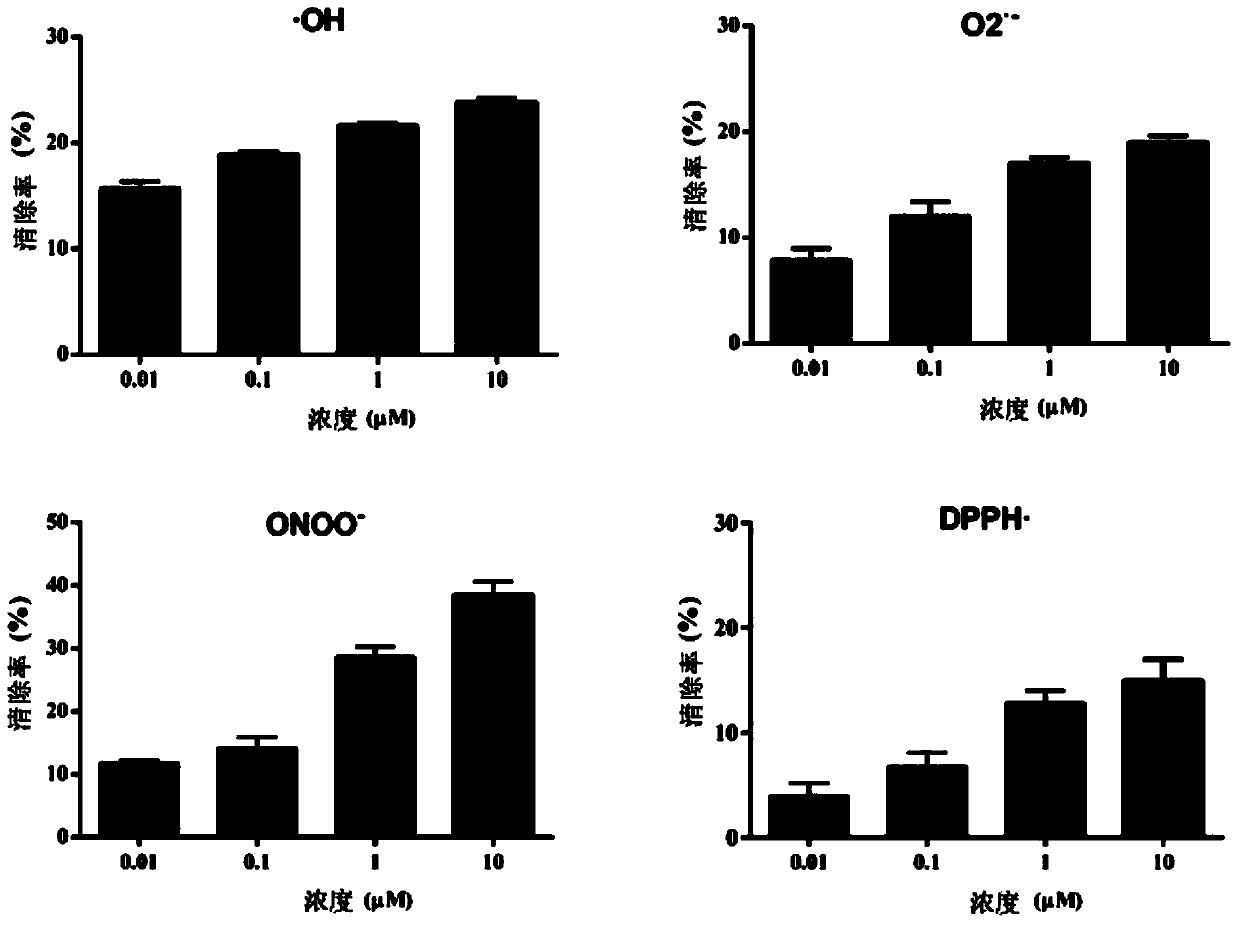
[0036] Embodiment 1. Andrographolide derivative AL-1 is respectively to hydroxyl free radical (OH), superoxide anion free radical (O2ˉ ), peroxynitrite ion (ONOOˉ) and 1,1-diphenyl in vitro The Scavenging Effects of Four Free Radicals of Radical-2-Phenylhydrazine Free Radical (DPPH)
[0037] Hydroxyl radical (OH): using o-phenanthroline-metal ion-H2O2, through the Fenton reaction (H 2 o 2 +Fe 2+ →·OH+H 2 O+Fe 3+ ) to generate hydroxyl radicals, prompting o-phenanthroline-Fe 2+ Oxidized to o-phenanthroline-Fe 3+ , causing the maximum disappearance of its aqueous solution at a wavelength of 440nm to measure its clearance rate. The specific steps are: add 300 μL of double-distilled water (blank control) or different concentrations of andrographolide derivative AL-1 (dissolved in DMSO to form a 10 mM stock solution, and then diluted with double-distilled water to 0.01 μM, 0.1μM, 1μM, 10μM), add 50μL of1.0mM o-phenanthroline (dissolve 1.0mM in 50mM NaCl solution), then add 1...
Embodiment 2
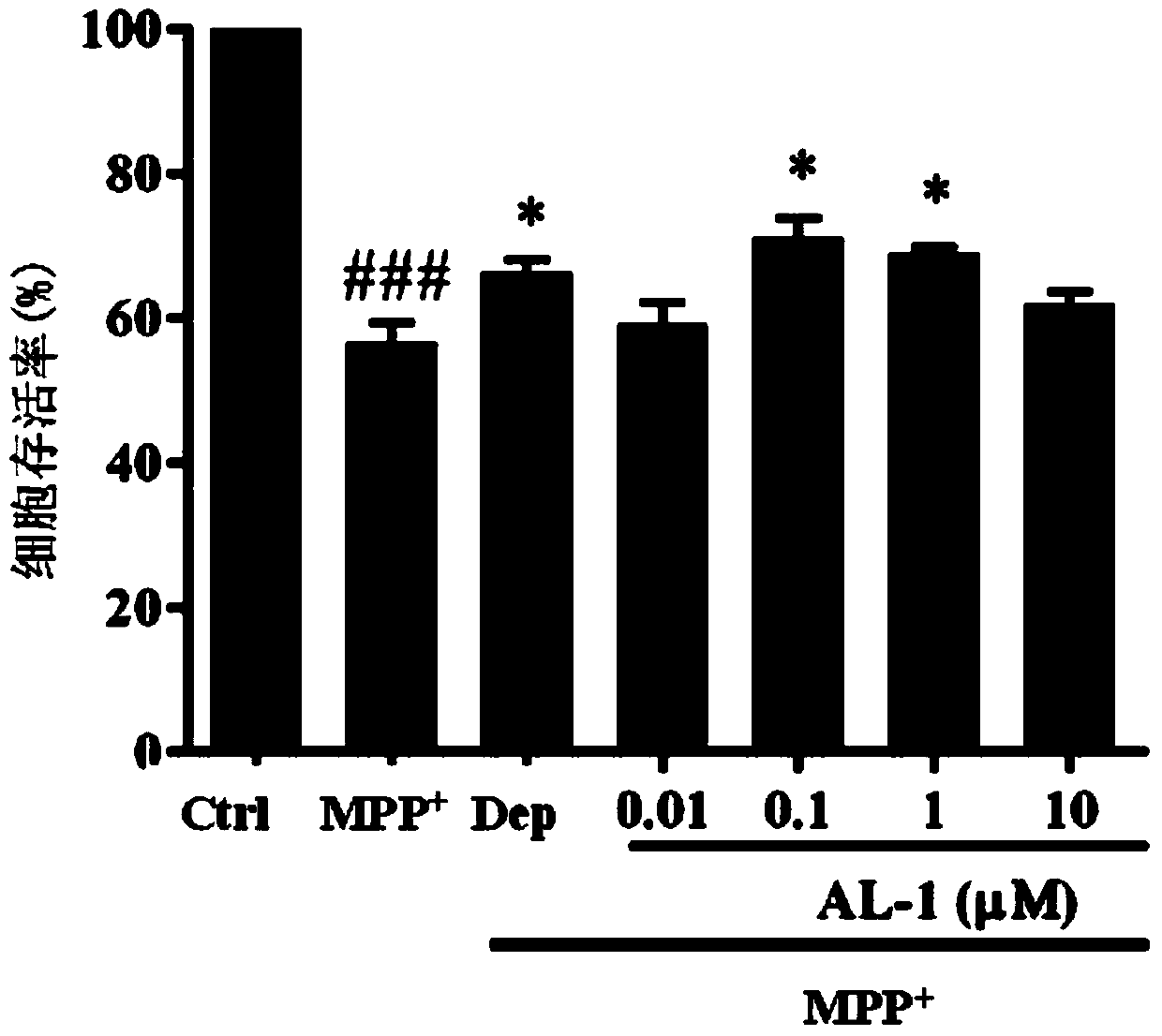
[0043] Embodiment 2. andrographolide derivative AL-1 is to MPP in vitro + Protective activity of induced SH-SY5Y cell injury
[0044] SH-SY5Y cells were digested and seeded in 96-well plates (2×10 4per well), 100 μl per well. After the cells were cultured in the incubator for 24 hours, the culture medium was discarded. The culture medium (containing 2% FBS) containing different concentrations of andrographolide derivative AL-1 (0.01 μM, 0.1 μM, 1 μM, 10 μM) was protected for 2 hours, and then 2 mM MPP was added + Incubate for 24 hours, then add a new medium solution containing 5mg / ml MTT after aspirating the old medium. After incubating in the incubator for 4 hours in the dark, the culture medium was sucked off, and 150 μl DMSO was added to each well, shaken in the dark until the precipitate was completely dissolved, and the absorbance value at 570 nm was measured with a microplate reader. Taking the normal cell survival rate as 100%, the cell survival rate of different tr...
Embodiment 3
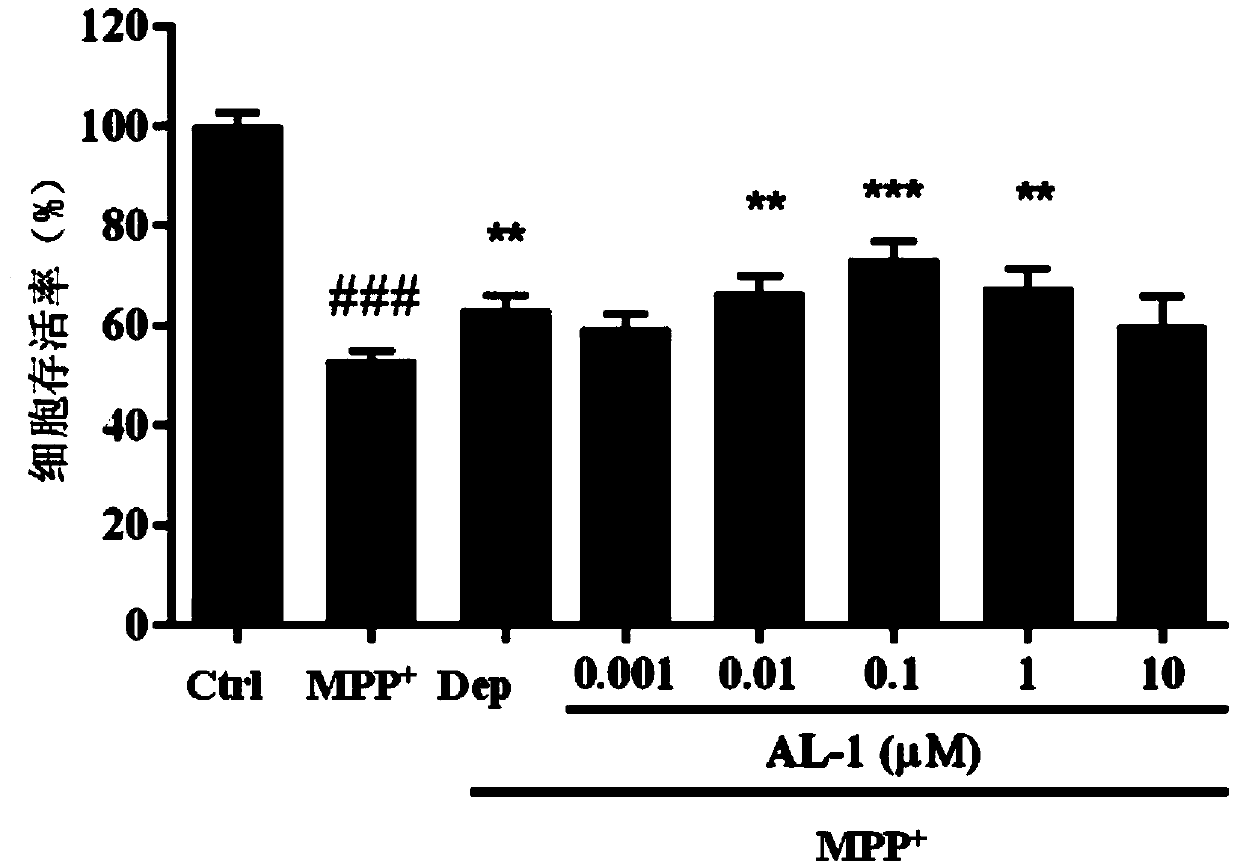
[0046] Example 3. Andrographolide derivative AL-1 to MPP + Protective effect of induced primary cultured cerebellar granule neurons
[0047] Primary cerebellar granule cells were extracted from 3-day-old neonatal mice (body weight 20-25g) and inoculated in 96-well plates (2×10 5 per well), 100 μl per well, after culturing in the incubator for 24 hours, add 10 μM cytarabine to the medium. After culturing to the eighth day, after adding different concentrations of andrographolide derivative AL-1 (0.01 μM, 0.1 μM, 1 μM, 10 μM) for protection, the absorbance of each group was measured by MTT method after induction with 200 μM MPP+ for 24 hours.
[0048] andrographolide derivative AL-1 on MPP + The experimental data of the neuroprotective effect of the induced cerebellar granule neuron cell injury model are as follows: image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com