High-activity benzoiso abscisic acid analogue and preparation method thereof
A high-activity, benzoisotropic technology, applied in botany equipment and methods, oxidative preparation of carboxylic acids, ozone oxidation preparation of carboxylic acids, etc., can solve the problems of 8'-hydroxyabscisic acid instability and low ring-closing activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
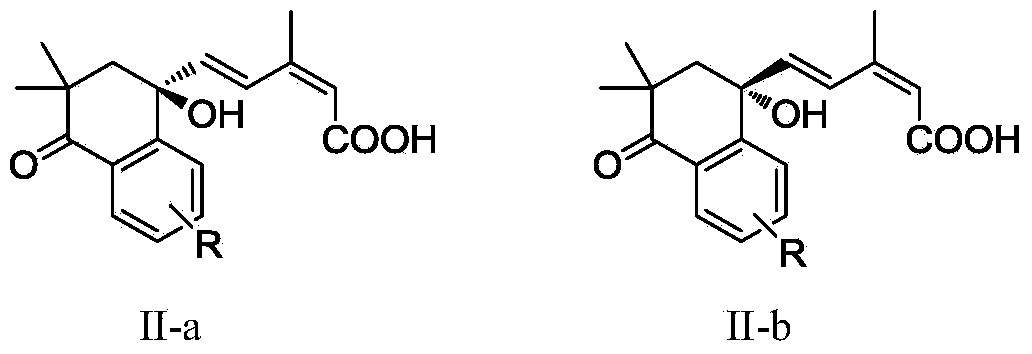
[0013] Embodiment 1: the synthesis of compound (II)
[0014] Step 1: Preparation of 2,2-dimethyl-3,4-dihydronaphthalene-1(2H)-one
[0015] Add NaH (11.6g, 0.34mol, 70%), tetralone (10.0g, 0.69mol) and 150mL anhydrous tetrahydrofuran into a dry 250mL three-necked flask, and stir at room temperature for 10 minutes to obtain a gray suspension. Then methyl iodide (11.1 mL, 178 mmol) was added dropwise to the above suspension, heated to 40° C. for 30 minutes after the dropwise addition, and then continued to stir at room temperature for 3 hours. After the reaction was completed, a small amount of water was slowly added dropwise to quench, extracted with ethyl acetate (3×20mL), the organic phases were combined, washed three times with saturated brine, dried over anhydrous magnesium sulfate, filtered, concentrated, and the concentrate was directly chromatographed (petroleum Ether: ethyl acetate = 10:1) to obtain 2,2-dimethyl-3,4-dihydronaphthalene-1(2H)-one (11.3, 95%) as a yellow o...
Embodiment 2
[0024] Example 2: Biological Activity of Benzoabscisic Acid Analogs
[0025] 1. Arabidopsis Seed Germination and Seedling Growth
[0026] Sample preparation: Preparation of (±)-ABA and test agent solution: Accurately weigh a certain amount of (±)-ABA and test agent, dissolve them in 1mL of anhydrous methanol respectively to obtain a 10mM mother solution, and store at -20°C light save. Pipette a certain amount of mother liquor with a pipette gun and dilute to the desired concentration with distilled water.
[0027] MS Medium (100 mL): Dissolve 0.44 g of MS519, 3.0 g of sucrose and 0.9 g of agar in 100 mL of distilled water.
[0028] When preparing the medium, first dissolve MS and sucrose in distilled water, and use KOH (2N) to adjust the pH value of the solution to 5.8-6.0. Divide into 75mL bottles, then accurately weigh 0.67g of agar and add to each bottle, cover with a parafilm and fix it with a rubber band. Put it into a high-temperature sterilization pot and sterilize ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com