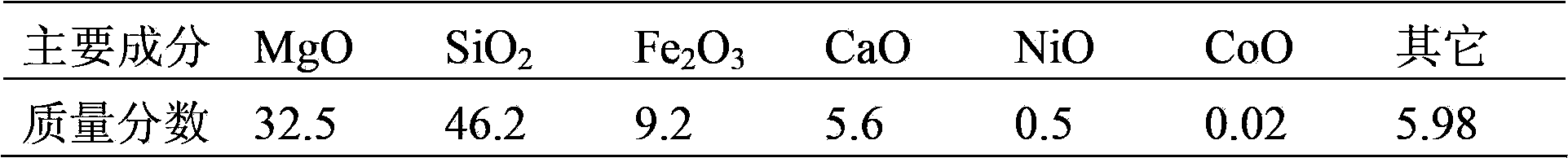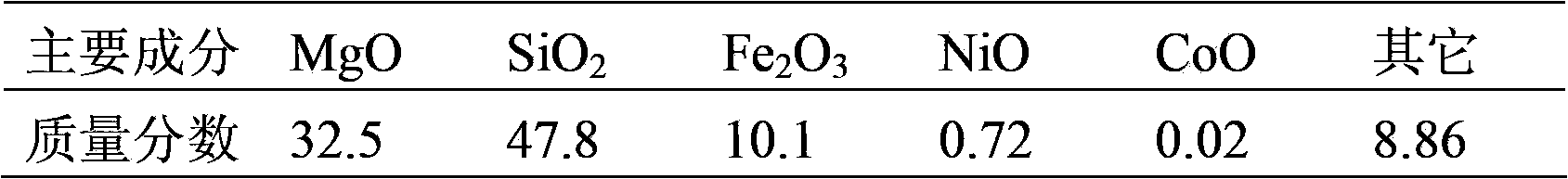Method for preparing magnesium oxide, nickel, cobalt and white carbon black through utilizing serpentine
A technology of serpentine and magnesium oxide, applied in chemical instruments and methods, magnesium oxide, silicon oxide, etc., can solve the problems of hindering the process of industrialization, incomplete product separation, and low magnesium leaching rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
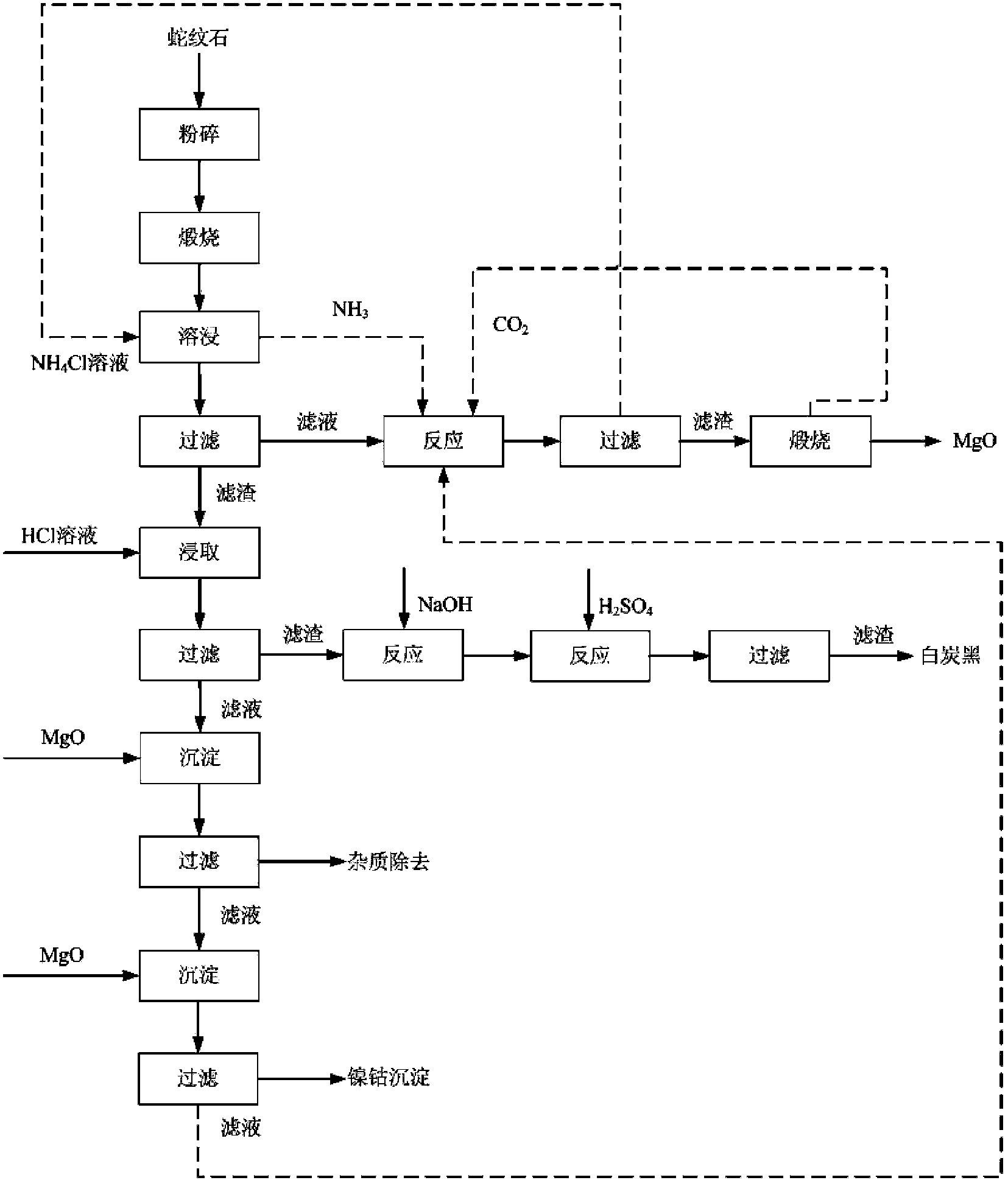
[0065] (1) Calcinate a serpentine ore in a muffle furnace for 240 minutes at a temperature of 650°C, and then grind it to obtain powdery particles with a particle size of 90-150 mesh. The chemical composition of the serpentine ore after calcination was analyzed and determined by chemical analysis (see Table 1).
[0066] (2) Configure 1000ml of 2mol / L ammonium chloride aqueous solution, put it in the reactor, control the temperature of the reactor at 105°C, weigh 80 grams of the powdery particles obtained in step (1) into the reactor, stir for 2 After an hour, cool down to room temperature naturally.
[0067] (3) filtering the magma obtained in step (2), separating and removing insoluble matter, the content of magnesium in the obtained magnesium chloride solution is 0.61mol / L;
[0068] (4) Place 500 mL of the magnesium chloride solution obtained in step (3) in a reaction vessel, control the temperature of the reaction vessel to 80°C, and pass through the solution with ammonia ...
Embodiment 2
[0076] (1) Calcinate a serpentine ore in a muffle furnace for 240 minutes at a temperature of 450°C, and then grind it to obtain powdery particles with a particle size of 45-65 mesh. The chemical composition of the serpentine ore after calcination was analyzed and determined by chemical analysis (see Table 2).
[0077] (2) Configure 1000ml of 2mol / L ammonium chloride aqueous solution, put it in the reactor, control the temperature of the reactor at 110°C, weigh 80 grams of the powdery particles obtained in step (1) into the reactor, stir for 2 After an hour, cool down to room temperature naturally.
[0078] (3) filtering the magma obtained in step (2), separating and removing insoluble matter, the content of magnesium in the obtained magnesium chloride solution is 0.60mol / L;
[0079] (4) Place 500 mL of the magnesium chloride solution obtained in step (3) into a reaction vessel, control the temperature of the reaction vessel to 85°C, and pass through the solution with ammonia...
Embodiment 3
[0087] (1) Calcinate a serpentine ore in a muffle furnace for 240 minutes at a temperature of 850°C, and then grind it to obtain powdery particles with a particle size of 65-90 mesh. The chemical composition of the serpentine ore after calcination was analyzed and determined by chemical analysis (see Table 3).
[0088] (2) Configure 1000ml of 2mol / L ammonium chloride aqueous solution, put it in the reactor, control the temperature of the reactor at 130°C, weigh 80 grams of the MgO powder obtained in step (1) into the reactor, stir for 2 After an hour, cool down to room temperature naturally.
[0089] (3) filtering the magma obtained in step (2), separating and removing insoluble matter, the content of magnesium in the obtained magnesium chloride solution is 0.63mol / L;
[0090] (4) Place 500 mL of the magnesium chloride solution obtained in step (3) in a reaction vessel, control the temperature of the reaction vessel to 80°C, and pass through the solution with ammonia and carb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com