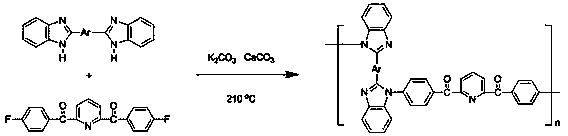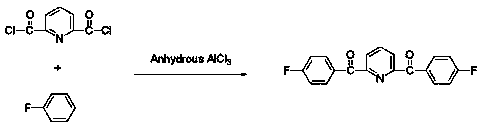N-substituted polybenzimidazole pyridine compound and preparation method thereof
A polybenzimidazole pyridine compound technology, which is applied in the field of N-substituted polybenzimidazole pyridine compounds and its preparation, can solve the problems of affecting heat resistance level and stability reduction, and achieve good solubility, high heat resistance level, The effect of simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0018] The preparation method of N-substituted polybenzimidazole pyridine compound of the present invention, comprises the following steps successively:
[0019] (1) Preparation of 2,6-bis(4-fluorobenzoyl)pyridine compound.
[0020] Under the condition of nitrogen protection, 2,6-bis(diformyl chloride)pyridine and fluorobenzene are used as monomers, and 2,6-bis(4-fluorobenzoyl)pyridine is obtained under the catalysis of anhydrous aluminum chloride The crude product can be purified to obtain 2,6-bis(4-fluorobenzoyl)pyridine compound.
[0021]
[0022] (2) Preparation of N-substituted polybenzimidazole pyridine compounds.
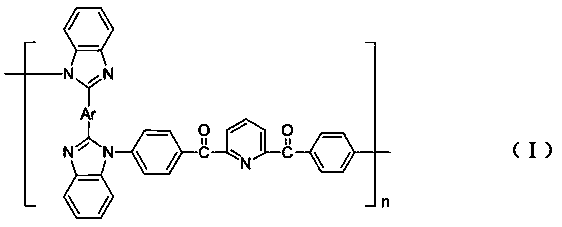
[0023] Under the condition of nitrogen protection, with the 2,6-bis(4-fluorobenzoyl)pyridine compound and bis(benzimidazole) compound prepared above as monomers, add sulfolane, chlorobenzene and calcium carbonate, in anhydrous Under the catalysis of potassium carbonate, an N-substituted polybenzimidazole pyridine compound is obtained.
[0024]
[002...
Embodiment 1
[0034] Example 1 Polycondensation synthesis of N-substituted polybenzimidazole pyridine compounds.
[0035] Include the following steps:
[0036] (1) In an ice-water bath, add 0.05 mol of 2,6-bis(diformyl chloride)pyridine, 0.65 mol of fluorobenzene, and 0.12 mol of anhydrous aluminum chloride powder into a 100 mL single-necked flask, stir for 12 h and then heat up to 95 o C and continue to react for 12 h. After the system temperature was slowly cooled to room temperature, the brown-red solution was slowly poured into deionized water, filtered, and washed with a large amount of deionized water to remove residual hydrogen chloride. Recrystallized twice with dimethylacetamide (DMAc) to give colorless crystals, vacuum 80 o C dried to give 2,6-bis(4-fluorobenzoyl)pyridine.
[0037]
[0038] (2) Nitrogen atmosphere, in a 25mL two-necked flask equipped with a condenser and a water separator, add bis(benzimidazole) intermediate- 0.01mol, 0.01mol of 2,6-bis(4-fluorobenzoyl)p...
Embodiment 2
[0041] Example 2 Polycondensation synthesis of N-substituted polybenzimidazole pyridine compounds.
[0042] Include the following steps:
[0043] (1) Nitrogen atmosphere, add bis(benzimidazole) compound- 0.01mol, 0.01mol of 2,6-bis(4-fluorobenzoyl)pyridine, 0.04mol of anhydrous potassium carbonate, 0.015mol of calcium carbonate, 10mL of sulfolane and 4mL of chlorobenzene. The system was raised from room temperature to 145°C and kept for 2h. Then the temperature was raised from 145°C to 180°C, and the reaction was continued for 4 hours, and the water in the system was removed by the water separator. Subsequently, the temperature was raised to 210° C., and the reaction was carried out for 6 hours. The system was slowly cooled to room temperature, poured into deionized water for precipitation, suction filtered, extracted with methanol, and then vacuum-dried at 100°C for 3 hours to obtain polybenzimidazole pyridine- .
[0044]
[0045] polybenzimidazole pyridine- It ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com