Catalyst for preparing carbon monoxide by decomposing carbon dioxide, as well as preparation method and application thereof
A carbon dioxide and carbon monoxide technology, applied in metal/metal oxide/metal hydroxide catalysts, carbon monoxide, physical/chemical process catalysts, etc. problems, to achieve the effect of easy industrial scale-up, lower decomposition temperature, and good performance repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
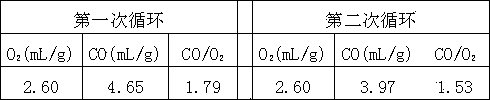
[0022] Example 1. Prepare a mixed aqueous solution according to the molar ratio of metal atoms as follows: Ce0.78Zr0.2Mg0.02 (each element is dissolved in deionized water in the form of nitrate to form a mixed aqueous solution), sodium hydroxide is made into 1 mol / L precipitant solution. At 80°C, the two solutions were co-precipitated. The precipitation process needs to be fully stirred to maintain pH = 10, aged at 80°C for 2 hours under stirring conditions, the precipitate was fully washed with deionized water, dried at 80°C and roasted at 800°C for 2 hours. , to obtain the metal oxide catalyst, the metal atom molar ratio of the catalyst is: n(Ce):n(Zr):n(Mg)=0.78:0.2:0.02, which is fully ground. The carbon dioxide decomposition cycle reaction of the catalyst of the present invention is carried out on a fixed-bed reactor. The specific reaction process is as follows: the catalyst is heated up to 1400 °C in an Ar atmosphere for thermal reduction reaction, and the reaction time...
Embodiment 2
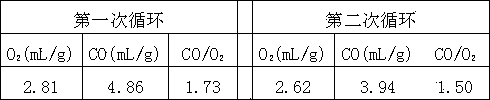
[0024]Example 2. Prepare a mixed aqueous solution according to the following molar ratio of metal atoms: Ce0.7Zr0.2Mg0.1 (each element is dissolved in deionized water in the form of nitrate to form a mixed aqueous solution), and potassium hydroxide is made into 0.5 mol / L precipitant solution. At 60°C, the two solutions were co-precipitated. The precipitation process needs to be fully stirred to maintain pH = 8, aged for 6 h under stirring at 60°C, the precipitate was fully washed with deionized water, dried at 100°C and roasted at 700°C After 4 h, the metal oxide catalyst was obtained. The metal atom molar ratio of the catalyst was: n(Ce):n(Zr):n(Mg) = 0.7:0.2:0.1, which was fully ground. The carbon dioxide decomposition cycle reaction of the catalyst of the present invention is carried out on a fixed-bed reactor. The specific reaction process is as follows: the catalyst is heated to 1400 °C in an Ar atmosphere for thermal reduction reaction, and the reaction time is 40 min; ...
Embodiment 3
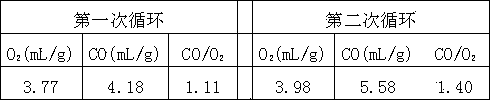
[0026] Example 3. Prepare a mixed aqueous solution according to the molar ratio of metal atoms as follows: Ce0.75Zr0.2Ca0.05 (each element is dissolved in deionized water in the form of nitrate to form a mixed aqueous solution), ammonia water is made into a 1.5 mol / L precipitant solution. At 50°C, the two solutions were co-precipitated. The precipitation process needs to be fully stirred, and the pH=12 is maintained. Aging is carried out at 50°C for 6 hours. The precipitate is fully washed with deionized water, dried at 120°C and roasted at 600°C for 4 hours. , to obtain the metal oxide catalyst, the metal atom molar ratio of the catalyst is: n(Ce):n(Zr):n(Ca)=0.75:0.2:0.05, which is fully ground. Carbon dioxide pyrolysis cycle reaction conditions are the same as example 2, and the obtained results are as follows:
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com