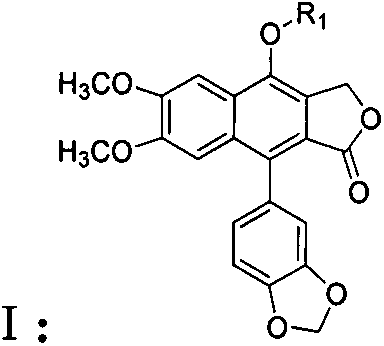
Lignin derivatives as well as preparation method and use thereof
A technology of lignans and derivatives, applied in botany equipment and methods, chemicals for biological control, biocides, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of intermediate 5-methyl-1,2,3-thiadiazole-4-carbonyl chloride:
[0033] Add 67 mmoles of 5-methyl-1,2,3-thiadiazole-4-carboxylic acid and 29 milliliters of thionyl chloride into a 100 milliliter three-necked round-bottomed flask, heat and reflux at 80 degrees Celsius for 6 hours, and depressurize Distill off excess thionyl chloride, and collect fractions at 94-96 degrees Celsius under reduced pressure at 2000 Pa to obtain 9.25 grams of light yellow product, yield 85%, intermediate 5-methyl-1,2,3-thiadiazole -4-Formyl chloride is sealed and stored in a desiccator for later use.
Embodiment 2
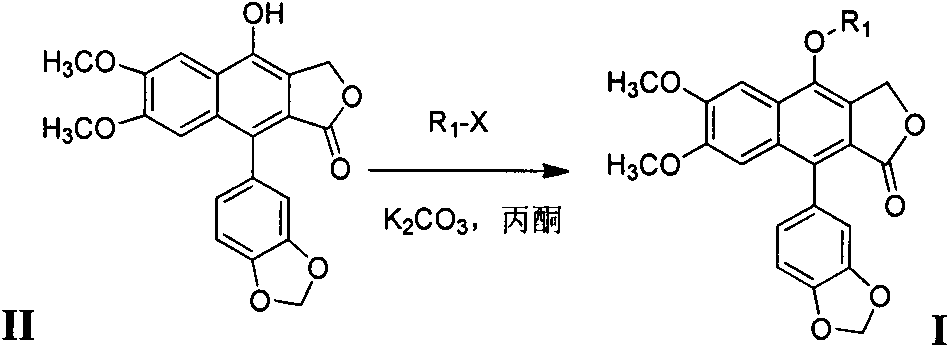
[0035] The preparation of lignan ether compound I:
[0036] Add 0.53 mmol of raw material II to a 50 ml round bottom flask, then add 20 ml of anhydrous acetone and 1.06 mmol of anhydrous potassium carbonate, stir at room temperature for 30 minutes, then add 0.53 mmol of halogenated hydrocarbon R 1 -X, heated to reflux for 12 hours, cooled the reaction system, filtered to remove the solid, and removed the solvent by rotary evaporation. The crude product was purified by 200-300 mesh silica gel column chromatography to obtain light yellow solid lignan ether compound I. The eluent was Petroleum ether at 61 to 93 degrees Celsius: ethyl acetate, the volume ratio is 3:1; the yield is calculated with the obtained pure product, and the yield is 58-64%. Its physical and chemical parameters and structural parameters are listed in Table 1. The halogenated hydrocarbon R 1 -X is selected from: 3-bromopropyne, 5-chloro-4-chloromethyl 1,2,3-thiadiazole, 1,1,3-trichloropropene, bromomethylcy...
Embodiment 3
[0038] Add 0.53 mmol of raw material II to a 50 ml round-bottomed flask, then add 20 ml of chloroform and 0.8 mmol of triethylamine, stir at room temperature for 10 minutes, and slowly add acid chloride R dropwise under ice-bath conditions. 1 -X in chloroform solution, stirred in ice bath for 0.5 hours, then reacted at room temperature for 3 hours, added ammonium chloride to stop the reaction, added 50 mL of chloroform to dilute, washed with saturated sodium bicarbonate (3×10 mL), anhydrous Dry over sodium sulfate and concentrate under reduced pressure. The crude product is purified by 200-300 mesh silica gel column chromatography to obtain light yellow solid lignan ester compound I. The eluent is petroleum ether at 61-93 degrees Celsius: ethyl acetate, according to the product The difference is that the volume ratio is 3:1; the yield is calculated with the obtained pure product, and the yield is 55.9%. Its physical and chemical parameters and structural parameters are listed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com