Preparation method of high-voltage nickel lithium manganate cathode material with porous morphology
A technology for lithium nickel manganese oxide and positive electrode materials, which is applied in the field of preparation of porous high-voltage lithium nickel manganese oxide positive electrode materials, can solve the problems of volume change, capacity attenuation, complex preparation process, etc., and achieve changing specific surface area and increasing magnification Performance, the effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0022] Specific embodiment one: this embodiment prepares lithium nickel manganese oxide material according to the following steps:
[0023] Weigh 0.03mol manganese carbonate and calcinate it in a muffle furnace at 800°C for 4 h to obtain porous manganese trioxide; mix the above manganese trioxide with 0.01 mol nickel acetate and 0.02 mol lithium nitrate by ball milling for 2 h to obtain the precursor The above precursors were placed in a muffle furnace, pre-calcined at 500°C for 4 hours, and calcined at 850°C for 10 hours to obtain a porous lithium nickel manganese oxide material. The SEM image is as follows figure 1 shown.
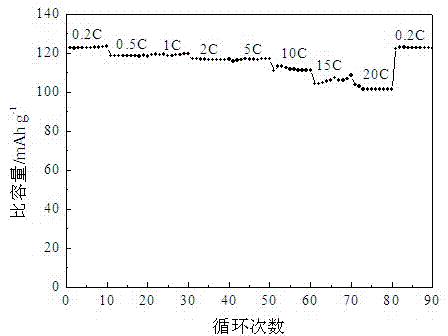
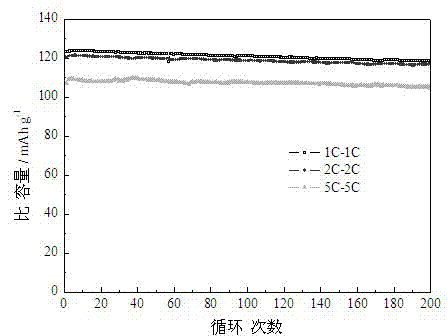
[0024] The pore volume of lithium nickel manganese oxide material prepared in this embodiment is 0.3 cm 3 / g, the specific surface area is 13m 2 / g. Such as Figure 2-3 As shown, the specific capacity can reach 101.3mAh / g when discharged at 20C, and the specific capacity is 118.2mAh / g, 116.7mAh / g and 104.6mAh / g after 200 charge-discharge cycles at 1C,...
specific Embodiment approach 2
[0025] Specific embodiment two: this embodiment prepares lithium nickel manganese oxide material according to the following steps:
[0026] Weigh 0.03mol of manganese carbonate and calcinate in a muffle furnace at 450°C for 4 h to obtain porous manganese dioxide; disperse the above manganese dioxide, 0.01mol of nickel nitrate, 0.01mol of lithium acetate and 0.012mol of lithium hydroxide into 20ml In ethanol, stir and volatilize ethanol at room temperature to obtain a precursor; put the above precursor in a muffle furnace, pre-calcine at 400 °C for 3 h, and calcinate at 800 °C for 12 h to obtain a porous lithium nickel manganese oxide material .
[0027] The pore volume of lithium nickel manganese oxide material prepared in this embodiment is 0.25m 3 / g, the specific surface area is 22m 2 / g. When discharged at 10C, the specific capacity can reach 111.3mAh / g, and after 200 charge-discharge cycles at 2C rate, the specific capacity is 117.9mAh / g, and the capacity retention rat...
specific Embodiment approach 3
[0028] Specific embodiment three: this embodiment prepares lithium nickel manganese oxide material according to the following steps:
[0029] Weigh 0.03mol of manganese oxalate and calcinate in a muffle furnace at 280°C for 6h to obtain porous manganese dioxide; disperse the above manganese dioxide, 0.005mol of nickel nitrate, 0.005mol of nickel formate, and 0.02mol of lithium formate into 15ml of ethanol , stirring and volatilizing ethanol at room temperature to obtain a precursor; placing the above precursor in a muffle furnace, pre-calcining at 400°C for 4 hours, and calcining at 800°C for 12 hours to obtain a porous lithium nickel manganese oxide material.
[0030] The pore volume of lithium nickel manganese oxide material prepared in this embodiment is 0.3m 3 / g, the specific surface area is 18m 2 / g. The specific capacity can reach 114.3mAh / g when discharged at 10C, and the specific capacity is 121.0mAh / g after 200 charge-discharge cycles at 2C rate, and the capacity r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com