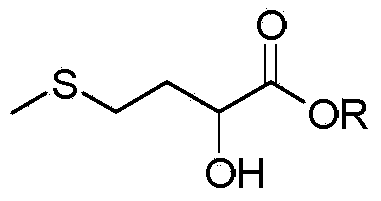
Preparation method of D,L-2-hydroxy-4-methylthiobutyrate
A technology of methylthiobutyrate and methylthiobutyronitrile, which is applied in the field of chemical industry, can solve the problems of increasing production cost and increasing separation and purification steps, and achieve the effects of improving production efficiency, reducing reaction steps and stabilizing properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
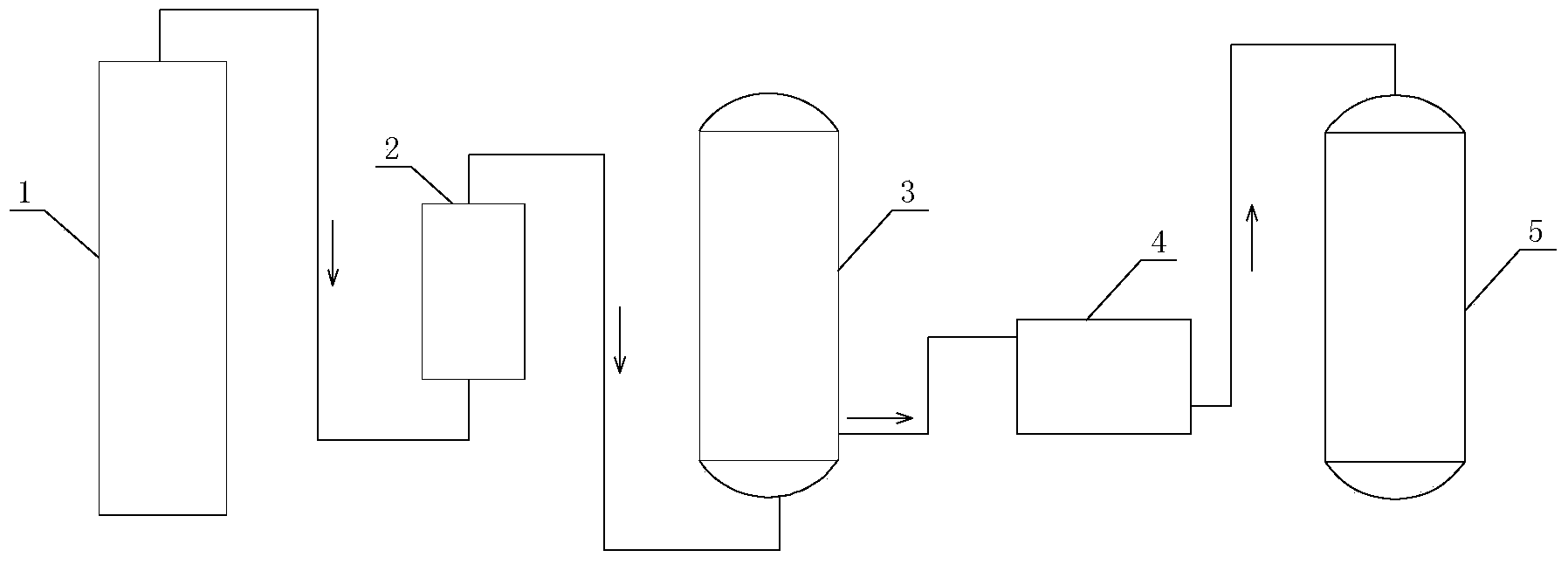
Image
Examples
Embodiment 1
[0044] The hydrogen cyanide gas mixture I from the hydrocyanic acid synthesis tower was tested. The composition of the hydrogen cyanide gas mixture I was: hydrogen cyanide gas 8.87%, water vapor 3.88%, ammonia 1.64%, hydrogen 1.13%, nitrogen 76.01%, oxygen 1.48%, carbon monoxide 5.67%, carbon dioxide 1.13%, methane 0.39%.
[0045] Hydrocyanic acid mixed gas I passes through a 75% sulfuric acid tower to absorb ammonia and water vapor in the mixed gas to obtain hydrocyanic acid mixed gas II. After testing, its composition is: hydrocyanic acid gas 9.35%, hydrogen 1.57%, Nitrogen is 79.44%, oxygen is 1.71%, carbon monoxide is 5.79%, carbon dioxide is 1.50%, and methane is 0.64%.
Embodiment 2
[0047] The hydrogen cyanide mixed gas II was passed into 223.3g of 94.5% methylthiopropionaldehyde, and the methylthiopropionaldehyde contained 3.3g pyridine. The reaction was performed under normal pressure, the reaction temperature was controlled to be 45°C, the aeration rate was 300L / min, the tail gas was absorbed by sodium hydroxide, and the residual amount of methylthiopropionaldehyde was monitored by HPLC. When the residual amount of methionylpropionaldehyde is less than 0.5%, it is the end of the reaction, and the flow can be stopped. A total of 270.64 g of light yellow liquid was obtained, the content of 2-hydroxy-4-methylthiobutyronitrile was 98%, and the residual hydrocyanic acid was 0.5%. Divide the obtained 2-hydroxy-4-methylthiobutyronitrile together with the reaction solution (ie 2-hydroxy-4-methylthiobutyronitrile reaction system) into two parts. Add 8.5g of water to one part and keep it at 3℃ for 120 days without decomposition; the other part is added with 8.5g...
Embodiment 3
[0049] The hydrogen cyanide mixed gas II was passed into 223.3g of 94.5% methylthiopropionaldehyde, and the methionylpropionaldehyde contained 2.2g pyridine and 10g water. Under 0.15MPa, the reaction temperature was controlled to 42°C, the aeration rate was 280L / min, the tail gas was absorbed by sodium hydroxide, and the residual amount of methylthiopropionaldehyde was monitored by HPLC. When the residual amount of methionylpropionaldehyde is less than 0.5%, it is the end of the reaction, and the flow can be stopped. A total of 279.54 g of light yellow liquid was obtained, the content of 2-hydroxy-4-methylthiobutyronitrile was 98%, and the residual hydrocyanic acid was 0.07%. The obtained 2-hydroxy-4-methylthiobutyronitrile together with the reaction liquid was equally divided into two parts. One part was stored at 3°C for 120 days without decomposition; the other part was acidified with 85% sulfuric acid to pH 3, and stored at 20°C for 130 days, 2-hydroxy-4-methylthiobutyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition efficiency | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
| decomposition efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com