Indirect process of preparing light calcium carbonate based on medium strengthening
A technology of light calcium carbonate and medium strengthening, which is applied in the direction of calcium carbonate/strontium/barium, etc., can solve the problems of complicated production process, unfriendly environment, and limited application, so as to reduce the difficulty of temperature control, wide range of process adaptability, wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
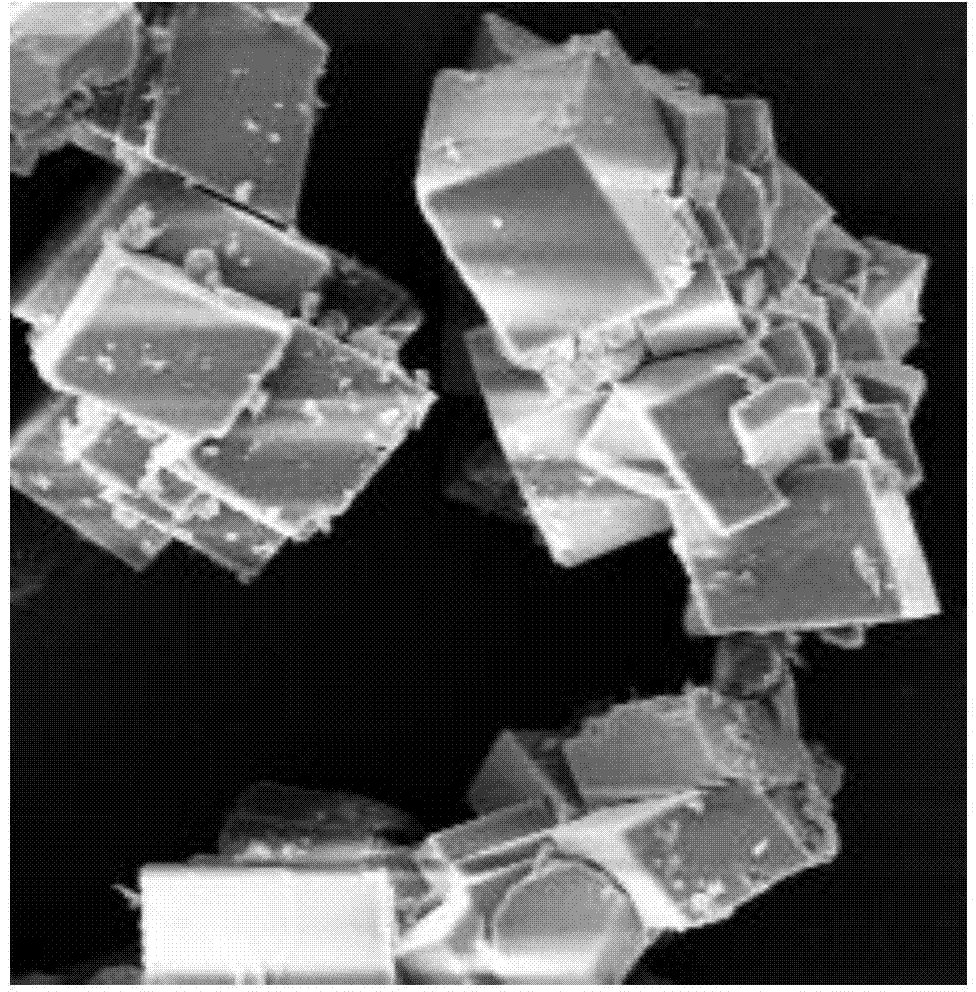
[0024] Mix 100ml of a calcium ion-rich solution with a calcium ion concentration of 2.4g / L, 100ml of tributylamine and 200ml of phase regulator n-butanol, and mechanically stir to make them evenly mixed. Before the reaction, the pH of the solution is stabilized at 10.0. Keep stirring, control the temperature at 27°C, and feed carbon dioxide to react, and the gas flow rate is 30ml / min. The reaction was stopped when the pH dropped to 8.0. The calcium carbonate after the reaction is filtered and dried, and the product is analyzed by a scanning electron microscope to obtain a crystal form of figure 1 The shape of vaterite calcium carbonate.
Embodiment 2
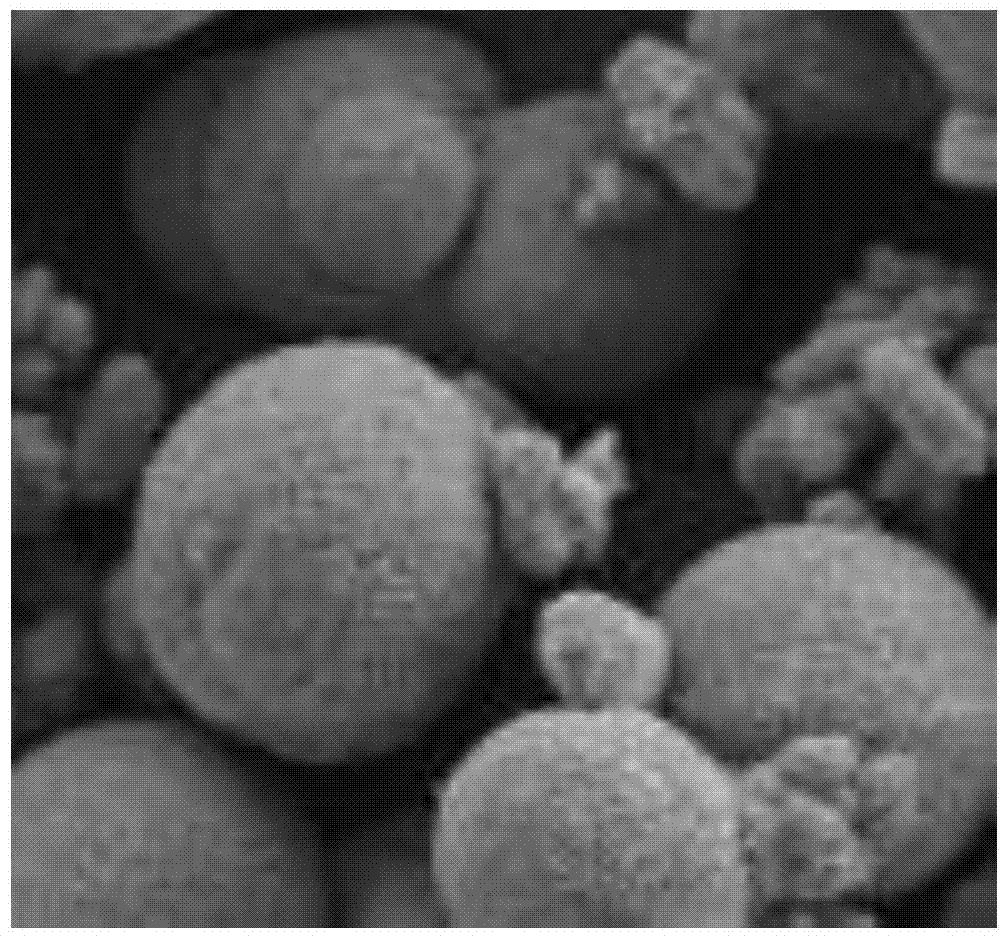
[0026] Mix 100ml of calcium ion-rich solution with a calcium ion concentration of 2.4g / L, 100ml of tributylamine and 200ml of phase regulator n-butanol, and mechanically stir to make them evenly mixed. Keep stirring, control the temperature at 15°C, feed carbon dioxide to react, the gas flow rate is 40ml / min, stop the reaction when the pH drops to 8.0. The calcium carbonate after the reaction is filtered and dried, and the product is analyzed by a scanning electron microscope and a laser particle size analyzer to obtain a crystal form of figure 2 Calcite-type calcium carbonate in shape with an average particle size of 0.79 microns.
Embodiment 3
[0028] Mix 100ml of calcium ion-rich solution with a calcium ion concentration of 5.0g / L, 100ml of tributylamine and 200ml of phase regulator n-butanol, and stir mechanically to make them evenly mixed. Keep stirring, control the temperature at 10°C, feed carbon dioxide to react, the gas flow rate is 40ml / min, the pH drops to 7.5, and stop the reaction. The reacted calcium carbonate is filtered and dried, and the product is analyzed by a laser particle size analyzer to obtain a crystal form of calcite-type calcium carbonate with an average particle size of 0.80 microns.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com