Rhodamine fluorescent probe with pseudo nucleic acid base as recognition site and preparation thereof and application to nucleotide image
A technology of identifying sites and nucleobases, applied in the field of fluorescent probes, can solve the problems of large light damage to cells, strong background signals, and it is difficult to accurately examine the physiological functions of cells, and achieve good cell condition and sensitive imaging effects. , the effect of good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 (preparation of probe RBS)
[0023] Dissolve 3.0g (27.5mM) 2,6-diaminopyridine in the first reaction kettle filled with 35mL concentrated phosphoric acid, heat to 90°C in an argon atmosphere, and weigh 3.70g (27.5mM) 4,4- Dimethoxy-2-butanone was placed in a constant pressure separatory funnel and added dropwise to the phosphoric acid solution of 2,6-diaminopyridine, heated to 115°C, stirred and reacted for 5 hours, cooled to room temperature, and added with 15% Phosphoric acid in the reaction solution was neutralized with ammonia water to pH=8, extracted 5 times with chloroform, the extracted chloroform solution was washed 3 times with saturated sodium chloride solution, dried with anhydrous magnesium sulfate, and the chloroform was rotary evaporated to obtain a black-red solid substance , recrystallized from toluene to obtain 2.30 g (52%) of light yellow powder intermediate 2-amino-methyl-1,8-naphthyridine (AMND); 1H NMR (CDCl 3 ,ppm):7.82(d,1H,J=4.0Hz),...
Embodiment 2
[0024] Example 2 (Measurement of Selective Response of Probes to Nucleotides)
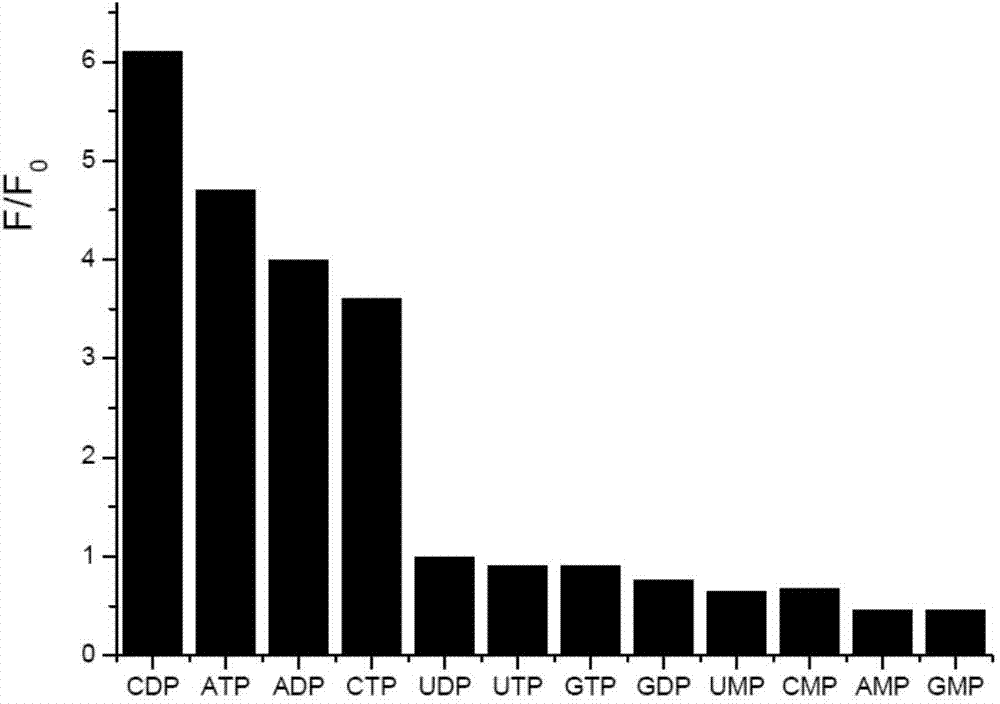
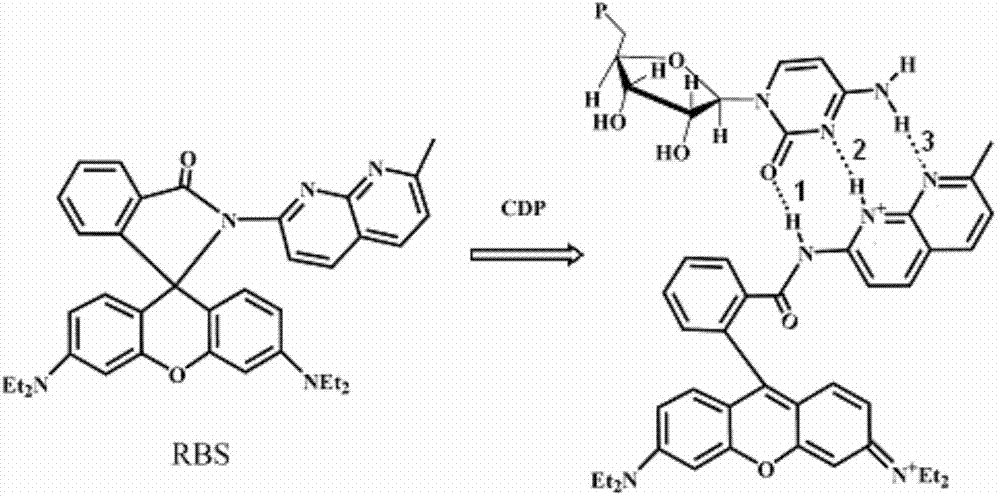
[0025] Weigh 30mg of the probe, and make it into a 10mM standard stock solution of acetonitrile, and make 10mM stock solution of nucleotides ADP, ATP, AMP, CDP, CTP, CMP, GDP, GTP, GMP, UDP, UTP, UMP respectively. Take 2 μL of the probe stock solution and add it to 2 mL of TRIS-HCl buffer solution with a concentration of 50 mM and a pH of 6.04, shake well and measure its fluorescence intensity (F 0), then add 30 μL of nucleotides, measure the fluorescence intensity (F) under the same conditions, and calculate the relative change value of the fluorescence intensity, the results are as follows figure 1 shown. The probes have different responses to all nucleotides, among which CDP, ATP, ADP and CTP cause obvious relative changes in fluorescence intensity, and CDP can form a strong complementary nucleotide-like base with naphthyridine in the probe. The triple hydrogen bond has the strongest effect, s...
Embodiment 3
[0026] Embodiment 3 (probe fluorescence intensity and absorbance change with CDP concentration)
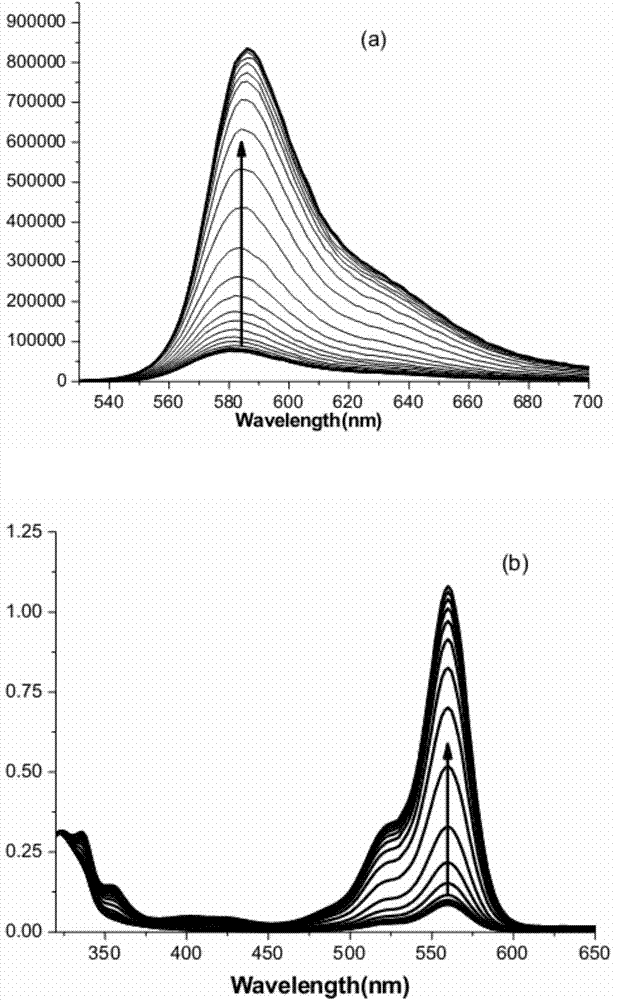
[0027] Weigh 10 mg of the probe, prepare a standard stock solution of 10 mM water (1), and then prepare a stock solution of 10 mM CDP (2). Measure 2 μL of the stock solution (1) and add it to 2 mL of TRIS-HCl buffer solution with a concentration of 50 mM and a pH of 6.04, shake well, add the calculated amount of stock solution (2), and prepare a standard test solution. The fluorescence intensity and absorbance were measured respectively, and the test results were as follows: image 3 shown. With the increase of CDP concentration, the fluorescence intensity and absorbance value of the probe gradually increased. Among them, Figure a is a graph of fluorescence change; Figure b is a graph of absorbance change, and the direction of the arrow in the figure indicates the direction of image intensity increase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com