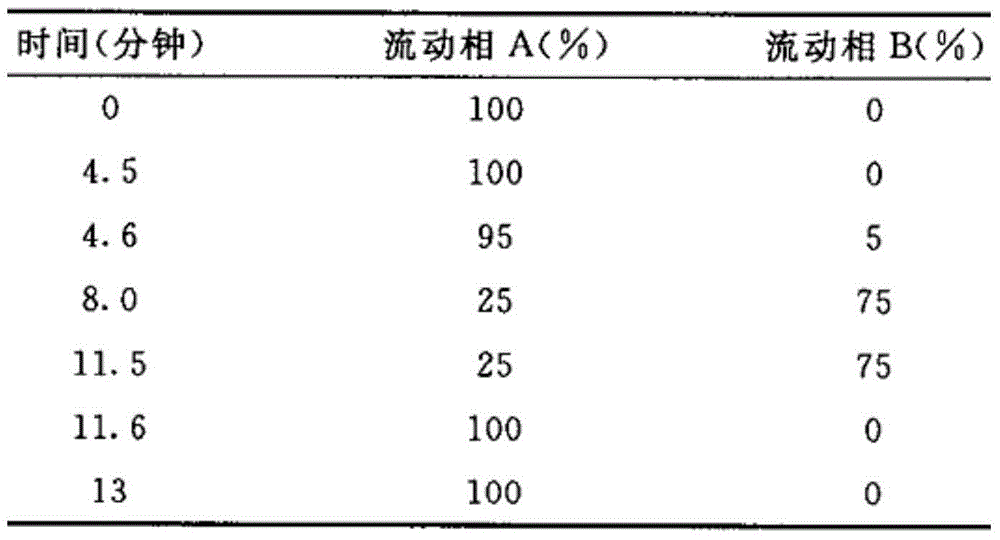
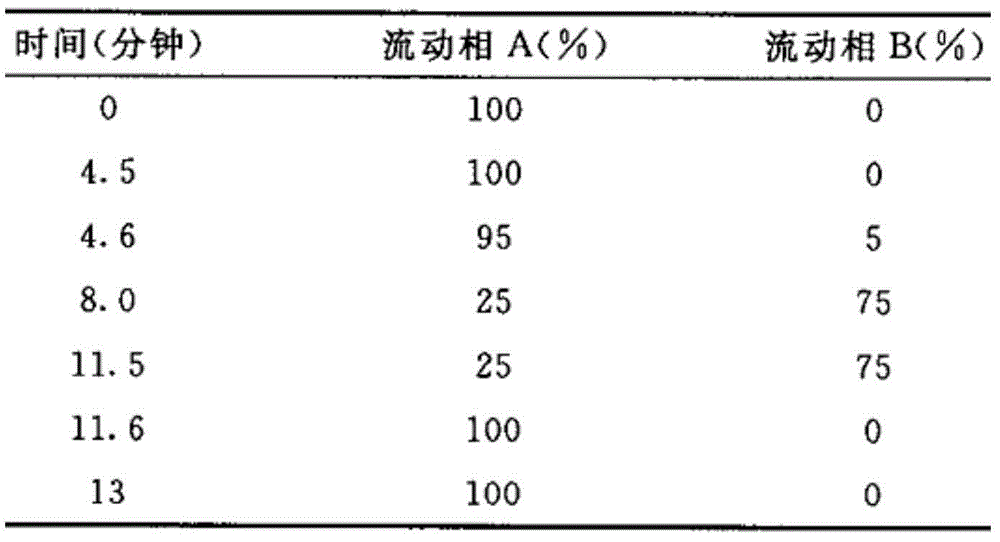
Simvastatin solid composition
A technology of simvastatin and composition, applied in the field of solid pharmaceutical composition and preparation thereof, can solve the problems of increased gastrointestinal tract burden, unstable tablet weight, decreased content and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Embodiment 1: Preparation of solid pharmaceutical composition of the present invention
[0086] formula:
[0087] Element
[0088] Preparation method: crush each material and pass through a 80-mesh sieve. Mix simvastatin, citric acid, and BHA thoroughly; spread the mixture in a closed container, place 50% ethanol at the bottom of the container and saturate the air in the container with the ethanol in advance, and seal the material in the container During this period, the ethanol saturation state is still maintained; the sealed container is placed at room temperature for 24 hours; then the mixture of the three materials is dried at a temperature of 55 °C until the moisture content is lower than 2%; then the dry mixture is fully mixed with the remaining materials , dry granulation (compression of large tablets, and then broken into granules suitable for tableting), to obtain the final mixture, a part (about 2 / 3) of the final mixture is compressed into tablets...
Embodiment 2
[0095] Embodiment 2: Preparation of solid pharmaceutical composition of the present invention
[0096] Recipe (amount per tablet):
[0097] Element
[0098] Preparation method: crush each material and pass through a 80-mesh sieve. Thoroughly mix simvastatin, citric acid, and BHA; spread the mixture in a closed container, place 40% ethanol at the bottom of the container and saturate the air in the container with the ethanol in advance, and seal the material in the container During this period, the ethanol saturation state is still maintained; the sealed container is placed at room temperature for 24 hours; then the mixture of the three materials is dried at a temperature of 50 ° C until the moisture content is lower than 2%; then the dry mixture is fully mixed with the remaining materials , dry granulation (compressing large tablets, and then crushing into granules suitable for tabletting) to obtain the final mixed material, which is compressed into tablets, and t...
Embodiment 3
[0101] Embodiment 3: Preparation of solid pharmaceutical composition of the present invention
[0102] Recipe (amount per tablet):
[0103] Element
[0104] Preparation method: crush each material and pass through a 80-mesh sieve. Thoroughly mix simvastatin, citric acid, and BHA; spread the mixture in a closed container, place 60% ethanol at the bottom of the container and saturate the air in the container with the ethanol in advance, and seal the material in the container During this period, the ethanol saturation state is still maintained; the sealed container is placed at room temperature for 30 hours; then the mixture of the three materials is dried at a temperature of 60 ° C until the moisture content is lower than 2%; then the dry mixture is fully mixed with the remaining materials , using 75% ethanol as a wetting agent for wet granulation, drying the wet granules to obtain a final blended material, which is compressed into tablets, and the composition can ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com