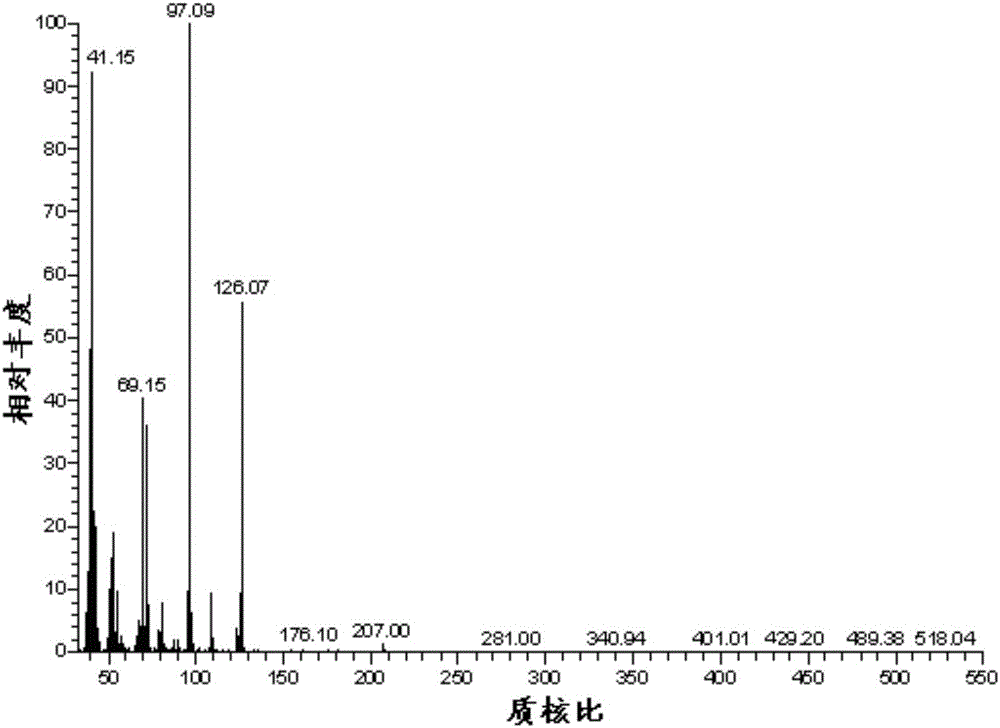
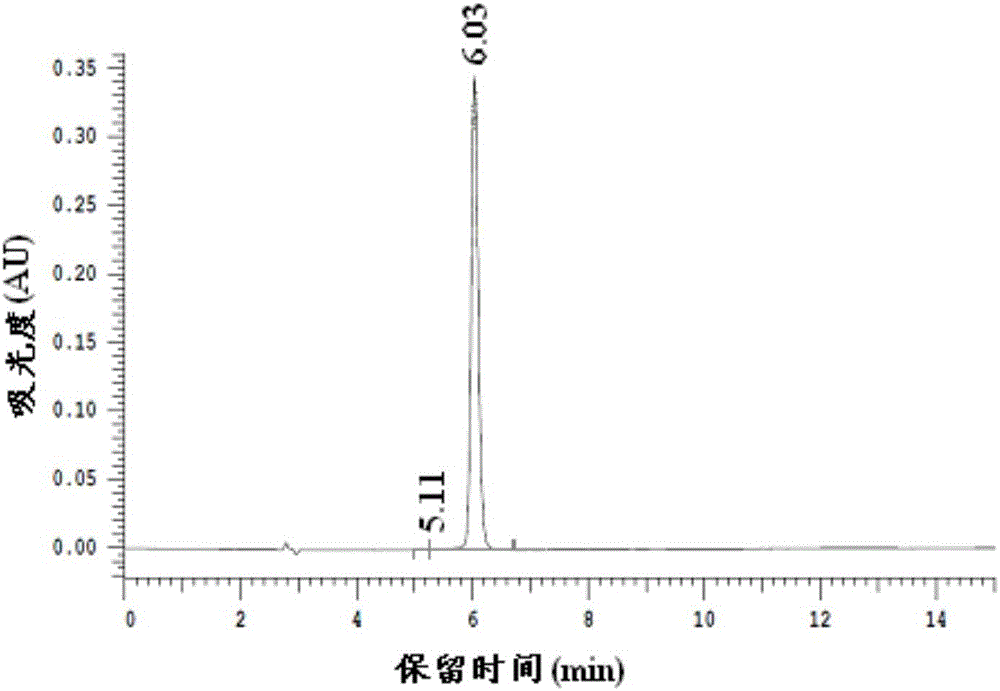
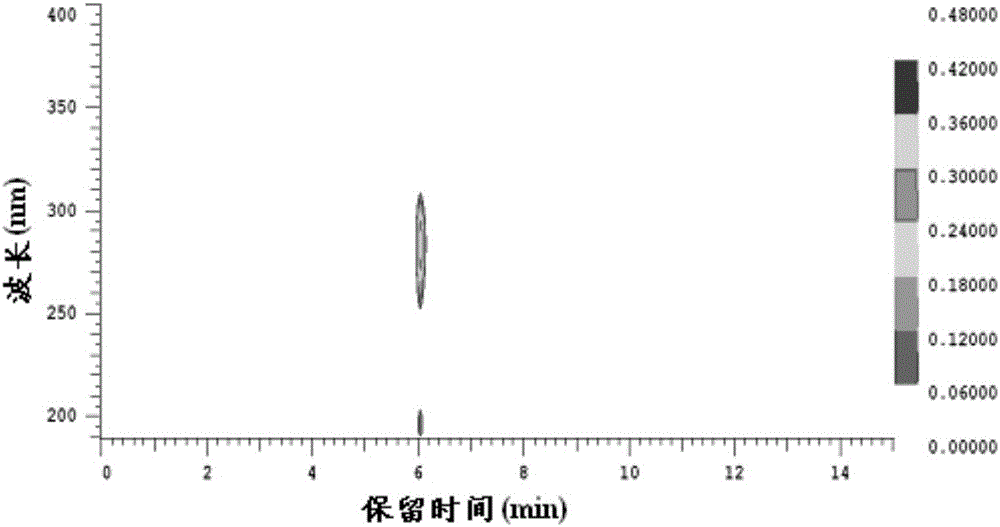
A kind of preparation method of 2,5-dimethylfuran
A technology of dimethyl furan and hydroxymethyl furfural, applied in directions such as organic chemistry, can solve problems such as low application value and low DMF yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The invention provides a kind of preparation method of 2,5-dimethylfuran, comprising the following steps:
[0037] Under the action of a nickel-based metal catalyst, 5-hydroxymethylfurfural is hydrogenolyzed in a solvent to obtain 2,5-dimethylfuran;
[0038] The nickel-based metal catalyst is a supported bimetallic catalyst;
[0039] The effective active components of the nickel-based metal catalyst include nickel and tungsten.
[0040] The method provided by the invention adopts a nickel-based metal catalyst with nickel and tungsten as active ingredients to catalyze HMF for hydrogenolysis to obtain DMF. The nickel component has a good hydrogenation ability and can hydrogenate aldehyde groups into methylol groups. group; the tungsten component has good Lewis acidity, which can promote the breakage of the carbon-oxygen bond in the hydrogenolysis process of HMF, and convert the methylol group into a methyl group; under the dual action of nickel and tungsten, HMF can be ...
Embodiment 1
[0090] Mix a certain proportion of nickel nitrate, ammonium metatungstate and activated carbon after weighing, so that the content of nickel in the resulting mixture is 7wt%, and the content of tungsten is 30wt%, and the mixture is placed in a dry flask with a stirring magnet Mix well, then add just enough distilled water to submerge the activated carbon into the flask.
[0091] Immerse the nickel nitrate, ammonium metatungstate and activated carbon in the above-mentioned flask for 12 hours, then place the impregnated product in a 90°C drying oven and dry the obtained dried product at 120°C for the first 6 Hours, the precursor of nickel-ditungsten carbide catalyst supported by activated carbon was obtained.
[0092] The precursor of the nickel-tungsten carbide catalyst obtained above with activated carbon as a carrier is 50mL / min at a hydrogen flow rate, and the nitrogen flow rate is 100mL / min for a second calcination. The process of the second calcination is: Heating at a he...
Embodiment 2
[0095] According to the technical scheme of embodiment 1, prepare the nickel-tungsten carbide catalyst with activated carbon as the carrier; the difference from embodiment 1 is to adjust the mixing ratio of nickel nitrate, ammonium metatungstate and activated carbon, so that the obtained nickel nitrate, metatungsten The content of nickel in the mixture of ammonium nitrate and activated carbon is 2wt%, and the content of tungsten is 30wt%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com