Amino/amine oxide modified perylene diimide derivative as well as preparation method and application thereof
A perylene diimide and derivative technology, applied in the field of organic optical devices, can solve the problems of low work function metal instability and unfavorable processing of organic semiconductor devices, etc., and achieve the effects of easy acquisition, high yield and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
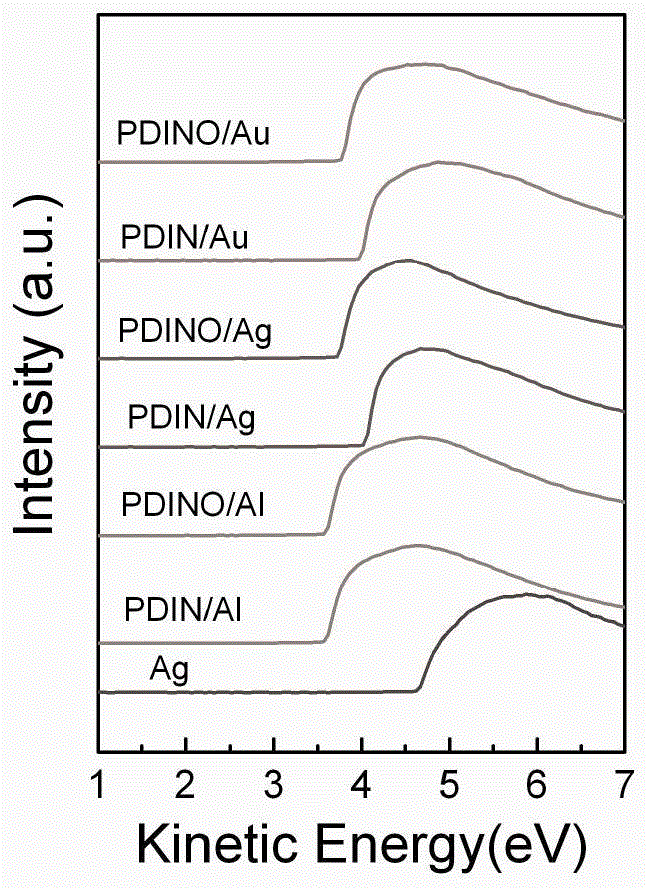
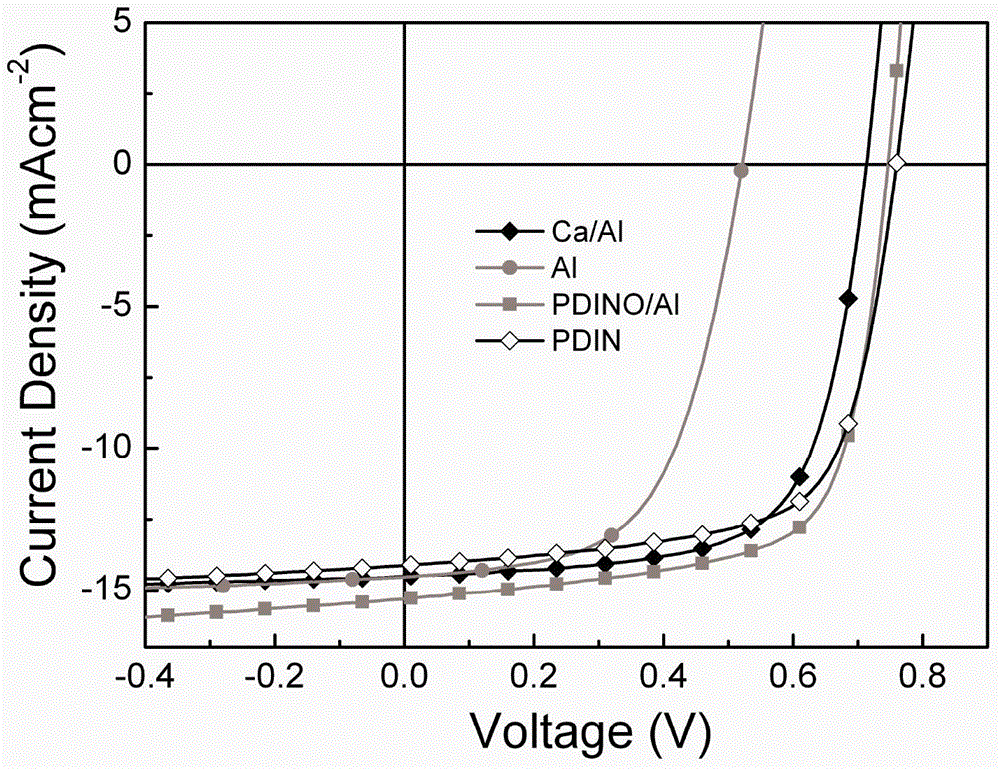
Image
Examples
Embodiment 1
[0077] Embodiment 1, the perylene diimide (that is, PDIN) of the amino group modification shown in the preparation formula III
[0078] Referring to the literature method (Angew Chem Int Ed2010, 49(8), 1485), under nitrogen atmosphere, pyrene tetraic anhydride (10 g, 25.6 mmol) and primary amine derivative N,N-dimethyl-1,3-di Aminopropane (equivalent to R in formula IX 1 for -ch 2 CH 2 CH 2 -,R 2 for -ch 3 ) (26.2 g, 141.6 mmol) in tert-butanol solution (500 mL), heated under reflux for 24 hours. Cooled to room temperature, the suspension was separated by filtration, and the filter cake was washed with ethanol and ether in turn. The product was obtained after drying. Yield 80%.
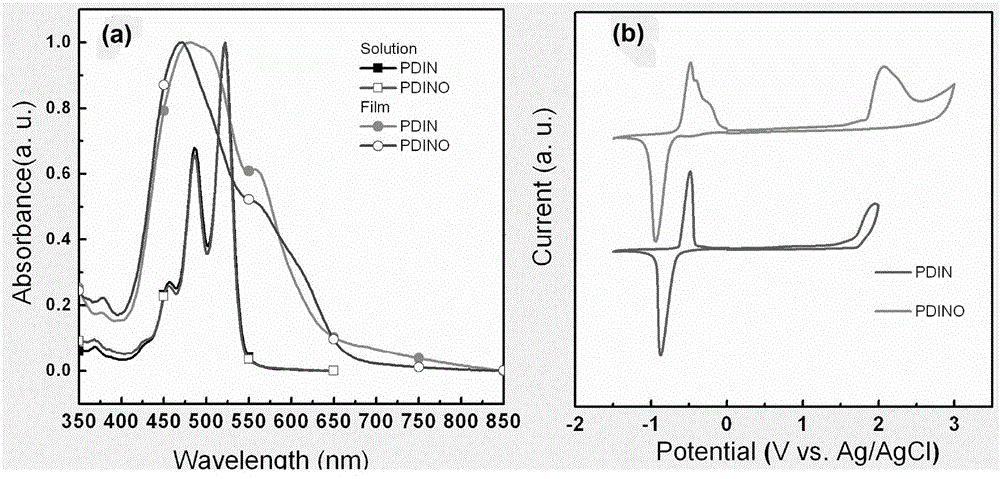
[0079] figure 1 The absorption spectrum of PDIN in a shows that there is a red shift in the absorption in the film compared to the solution, indicating that there is a strong intermolecular interaction between molecules in the solid state.
[0080] figure 1 The cyclic voltammogram of PDIN i...
Embodiment 2
[0085] Example 2. Preparation of amine group-modified perylene diimide shown in formula IV
[0086] With reference to the preparation method of formula III in Example 1, under nitrogen atmosphere, pyrene tetraic anhydride (10 g, 25.6 mmol) and primary amine derivative N,N-diethylethylenediamine (equivalent to formula IX, R 1 for -ch 2 CH 2 -,R 2 for -ch 2 CH 3 ) (16.45 g, 141.6 mmol) in tert-butanol solution (500 mL), heated under reflux for 24 hours. Cooled to room temperature, the suspension was separated by filtration, and the filter cake was washed with ethanol and ether in turn. The product was obtained after drying. Yield 80%.
[0087] The structural confirmation of this product is shown below: 1 H-NMR (400MHz, CF3COOD, δ, ppm): 8.90(4H), 8.95(4H), 4.89(4H), 3.80(4H), 3.60(8H), and 1.60(12H).
Embodiment 3
[0088] Example 3. Preparation of amine group-modified perylene diimide shown in formula V
[0089] Referring to the preparation method of formula III in Example 1, under nitrogen atmosphere, pyrene tetraic anhydride (10 g, 25.6 mmol) and primary amine derivative N,N-dimethylethylenediamine (equivalent to formula IX, R 1 for -ch 2 CH 2 -,R 2 for -ch 3 ) (20.0 g, 128.1 mmol) in tert-butanol solution (500 mL), heated under reflux for 24 hours. Cooled to room temperature, the suspension was separated by filtration, and the filter cake was washed with ethanol and ether in turn. The product was obtained after drying. Yield 80%.
[0090] The structural confirmation of this product is shown below: 1 H-NMR (400 MHz, CF3COOD, δ, ppm): 8.90 (4H), 8.95 (4H), 4.89 (4H), 3.80 (4H), 2.24 (6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com