Synthetic method of substituted benzamidine compound
A synthetic method and compound technology, applied in organic chemistry and other fields, can solve the problems of long synthetic route and complicated preparation of raw materials, and achieve the effects of low production cost, easy operation of reaction and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
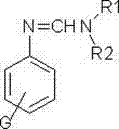
[0045] A kind of synthetic method of substituted benzamidine compound, the structural formula of described substituted benzamidine compound is as follows:
[0046]
[0047] where G is H;
[0048] Both R1 and R2 are ethyl groups.
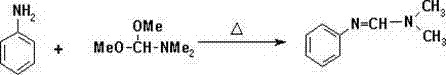
[0049] The synthetic method of above-mentioned substituted benzamidine compound, specifically comprises the following steps:
[0050] (1) In 20ml of organic solvent, mix 1g of intermediate II with 1.35g of POCl 3 In the presence of 2.30 g of alkali, control the reaction temperature to 40° C. and carry out the reaction for 1 h to obtain a solution containing intermediate III;
[0051] Described organic solvent is toluene;
[0052] Described alkali is triethylamine;
[0053] The general structural formula of the intermediate II is:
[0054] , where G is H;
[0055] Intermediate II, POCl used in the reaction process 3 , the amount of alkali and organic solvent, according to intermediate II: POCl 3 : Alkali: organic solvent is calculated acc...
Embodiment 2
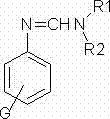
[0066] A kind of synthetic method of substituted benzamidine compound, the structural formula of described substituted benzamidine compound is as follows:
[0067]
[0068] Wherein G is 4-methyl;
[0069] Both R1 and R2 are ethyl groups.
[0070] The synthetic method of above-mentioned substituted benzamidine compound, specifically comprises the following steps:
[0071] (1) In 10ml of organic solvent, mix 0.5g of intermediate II with 0.74g of POCl 3 In the presence of 2.46 g of alkali, control the reaction temperature to 50° C. and carry out the reaction for 0.5 h to obtain a solution containing intermediate III;
[0072] Described organic solvent is benzene;
[0073] Described alkali is diisopropylamine;
[0074] The general structural formula of the intermediate II is:
[0075] , wherein G is 4-methyl;
[0076] Intermediate II, POCl used in the reaction process 3 , the amount of alkali and organic solvent, according to intermediate II: POCl 3 : Alkali: organic ...
Embodiment 3
[0087] A kind of synthetic method of substituted benzamidine compound, the structural formula of described substituted benzamidine compound is as follows:
[0088]
[0089] Wherein G is 4-Cl;
[0090] R1 and R2 form a six-membered ring with the nitrogen atom to which they are attached:
[0091] .
[0092] The synthetic method of above-mentioned substituted benzamidine compound, specifically comprises the following steps:
[0093] (1) In 10ml of organic solvent, mix 0.5g of intermediate II with 0.98g of POCl 3 In the presence of 1.79 g of base, control the reaction temperature to 40° C. and carry out the reaction for 1 h to obtain a solution containing intermediate III;
[0094] Described organic solvent is xylene;
[0095] Described alkali is triethylamine;
[0096] The general structural formula of described intermediate II p-chloroformanilide is:
[0097] , wherein G is 4-Cl;
[0098] Intermediate II, POCl used in the reaction process 3 , the amount of alkali ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com