Andrographolide-19-amide derivative, preparation method and its use in medicine
A technology of andrographolide and amide derivatives, which is applied in the fields of antineoplastic drugs, drug combinations, organic chemistry, etc., and can solve the problem of no compound pharmacological activity test, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
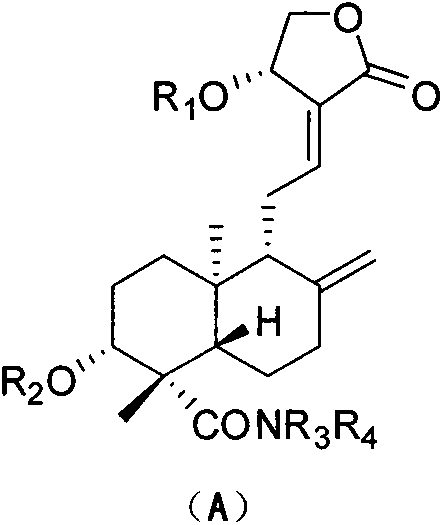
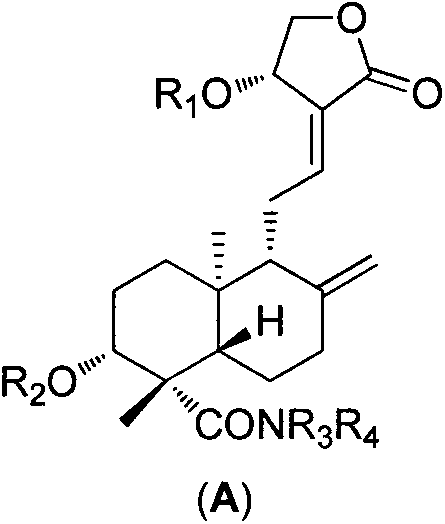
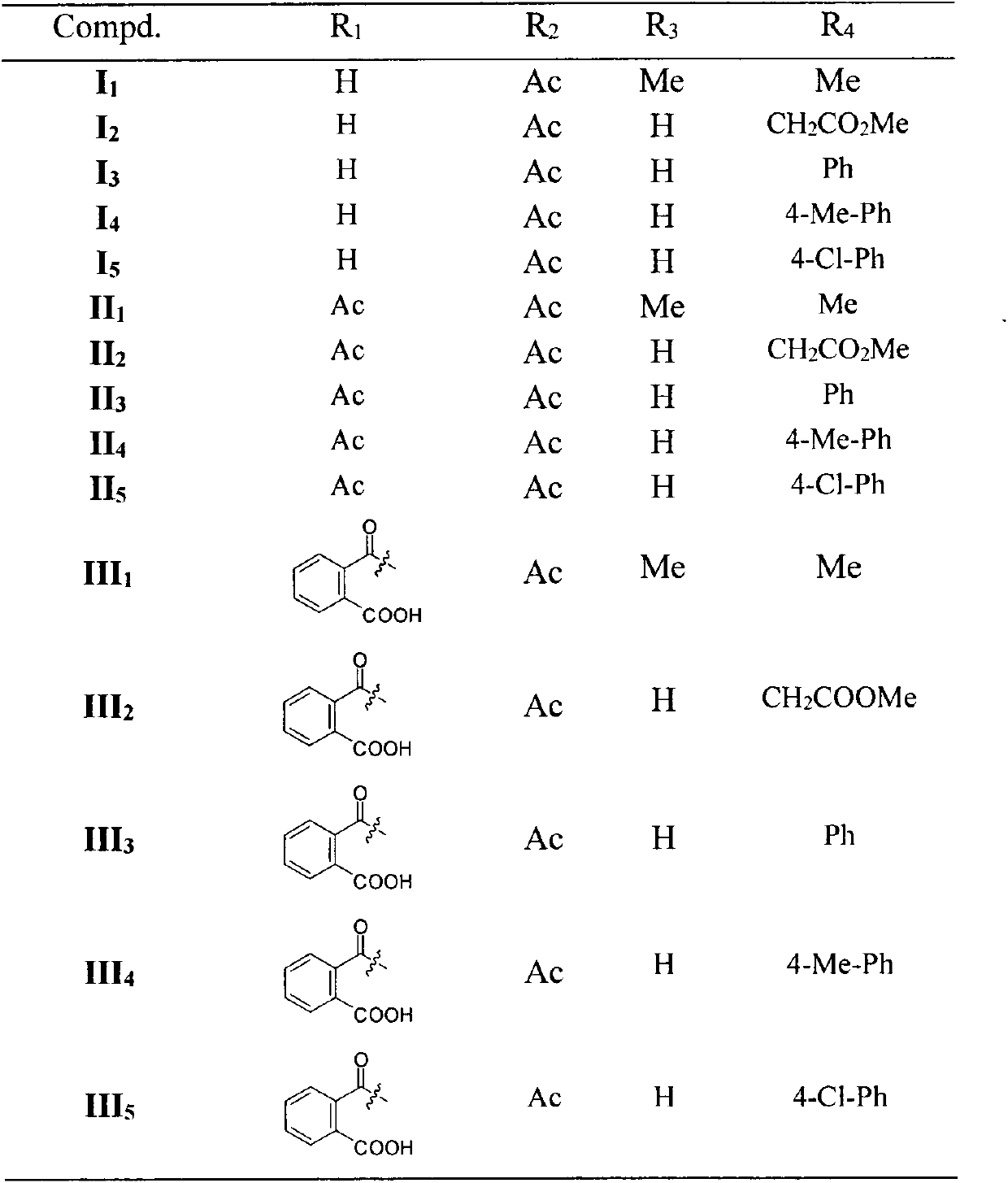
[0051] 3, Preparation of 19-isopropoxy-andrographolide (2)
[0052] Dissolve andrographolide (10.00g, 28.54mmol) in dry toluene / DMSO (200ml / 27ml), add 2,2-dimethoxypropane (14ml), p-toluenesulfonic acid (catalytic amount), 80°C Stir for about 1.5 hours until the reaction is complete, cool to room temperature, add triethylamine (7ml) to quench the catalyst, add toluene (150ml) to the reaction solution and wash with water (3×300ml), separate the organic layer, and dry over anhydrous sodium sulfate. Concentrate the dry organic phase, add diethyl ether (30ml), stir, filter and dry with suction to obtain white solid particles (10.36g, 26.53mmol, yield 93%). mp: 160-161°C. 1 H-NMR (300MHz, CDCl 3 , ppm) δ: 6.96 (1H, t, J=6.4Hz), 5.03 (1H, d, J=5.9Hz), 4.91 (1H, s), 4.63 (1H, s), 4.46 (1H, dd, J =10.4, 6.1Hz), 4.26(1H, dd, J=1.9, 10.4Hz), 3.97(1H, d, J=5.8Hz), 3.50(1H, dd, J=3.4, 8.4Hz), 3.2(dd , 1H), 2.64-2.40(4H, m), 2.05-1.97(2H, m), 1.85-1.72(4H, m), 1.32-1.25(3H, m), 1.42(3H...
Embodiment 2
[0054] Preparation of 14-O-tert-butyldimethylsiloxane-andrographolide (4)
[0055] 3,19-isopropoxy-andrographolide (7.00g, 17.93mmol) was dissolved in dry DMF (50ml), and imidazole (2.32g, 34.06mmol), TBSCl (4.86g, 32.27mmol) were added under ice-cooling ), rose to room temperature after 20 min, and stopped the reaction after 10 hours. The reaction solution was diluted with water (20ml), extracted with ethyl acetate (3X30ml), and the organic layer was separated. The organic phase is directly concentrated without drying, and then the concentrated crude product 3,19-isopropoxy-14-tert-butyldimethylsilyl ether-andrographolide is dissolved in an aqueous solution of acetic acid at room temperature (acetic acid: water = 28ml: 12ml), after the completion of the reaction, add sodium bicarbonate powder and stir until no bubbles overflow, dilute the reaction solution with dichloromethane (80ml), separate the organic layer and successively wash with saturated sodium bicarbonate solution...
Embodiment 3
[0057] Preparation of 14-O-tert-butyldimethylsilyl ether-andrographolide-19-aldehyde (5)
[0058] 14-O-tert-butyldimethylsilyl ether-andrographolide (5.00g, 10.76mmol) was dissolved in dichloromethane (130ml), and TEMPO (0.17g, 1.08mmol) was added successively under ice-cooling, and the pH was Potassium carbonate-sodium bicarbonate buffer solution (130ml), TBAB (0.35g, 1.08mmol) and NCS (2.73g, 20.44mmol) of 8-9 were vigorously stirred for about 9 hours until the reaction was complete. After the reaction was complete, the organic layer was separated, the aqueous layer was extracted with dichloromethane (2×100ml), the organic layer was washed with saturated brine (2×300ml), and dried over anhydrous sodium sulfate. The dried organic phase was concentrated and separated by column chromatography (petroleum ether: ethyl acetate) to finally obtain a white powdery solid (4.53 g, 9.79 mmol, yield 91%). mp: 101-102°C. 1 H-NMR (300MHz, CDCl 3 , ppm) δ: 9.76(1H, s), 6.80(1H, t, J=5.4H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com