Method for preparing SrCuSi4O10 and BaCuSi4O10 blue pigments through hydrothermal technique
A blue pigment, technology of technology, applied in chemical instruments and methods, luminescent materials, alkaline earth metal silicates, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: SrCuSi 4 o 10 preparation (1)
[0041] Weigh 5.7g Na 2 SiO 3 9H 2 O (20mmol) and 40mL distilled water were mixed to form solution A, and concentrated hydrochloric acid was added to adjust the pH value to 8; weigh 1.334g SrCl 2 ·6H 2 O (5mmol) and 15mL of distilled water were mixed to form solution B. After solution A and solution B are mixed, add 0.4g CuO (5mmol) powder and stir (30 minutes), then use concentrated ammonia water to adjust the pH value of the solution to 11.5, then put it into a high-pressure reactor with a volume of 80mL, so that the filling degree reaches 80 %, react at 250°C for 24 hours after sealing. After the reactor was cooled, the blue powdery product was collected.
[0042] Figure 6 It is a comparison chart of the X-ray diffraction pattern of the reaction product in this example and the fitting data of the single crystal X-ray diffraction. From Figure 6 It can be seen from the figure that the X-ray diffraction pattern of t...
Embodiment 2
[0044] Example 2: SrCuSi 4 o 10 Preparation (2)
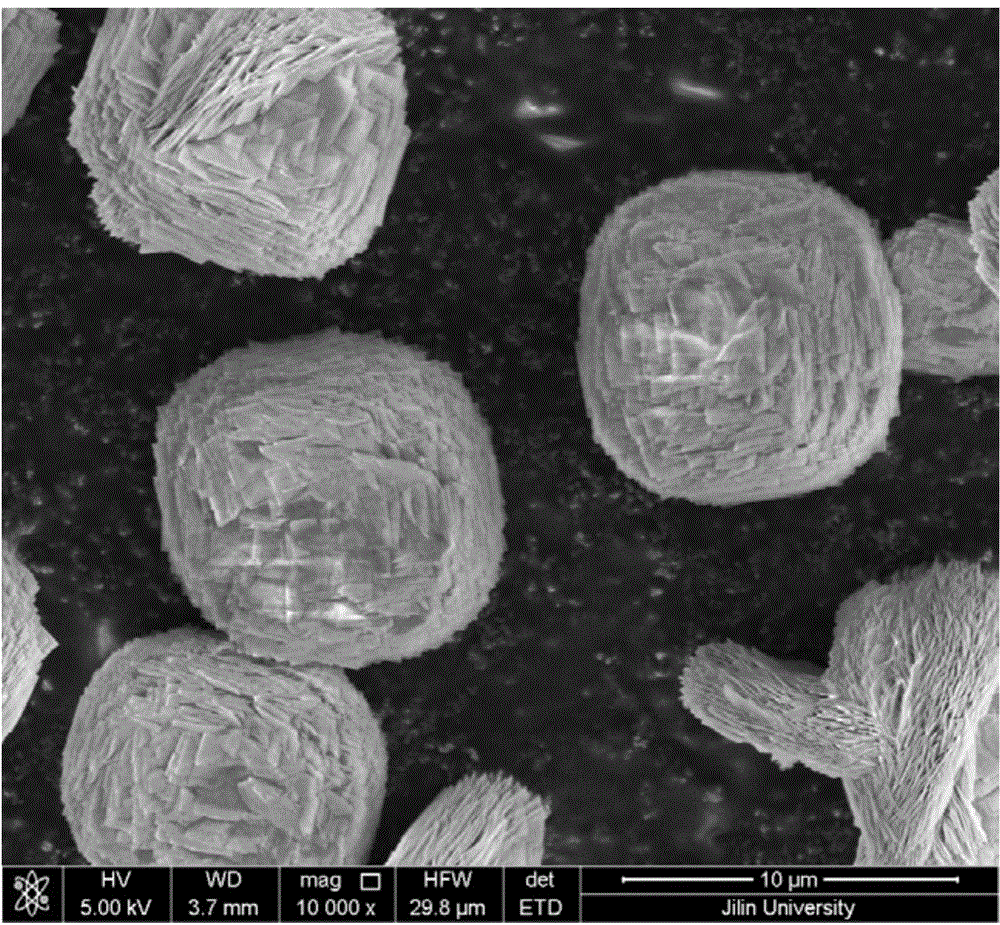
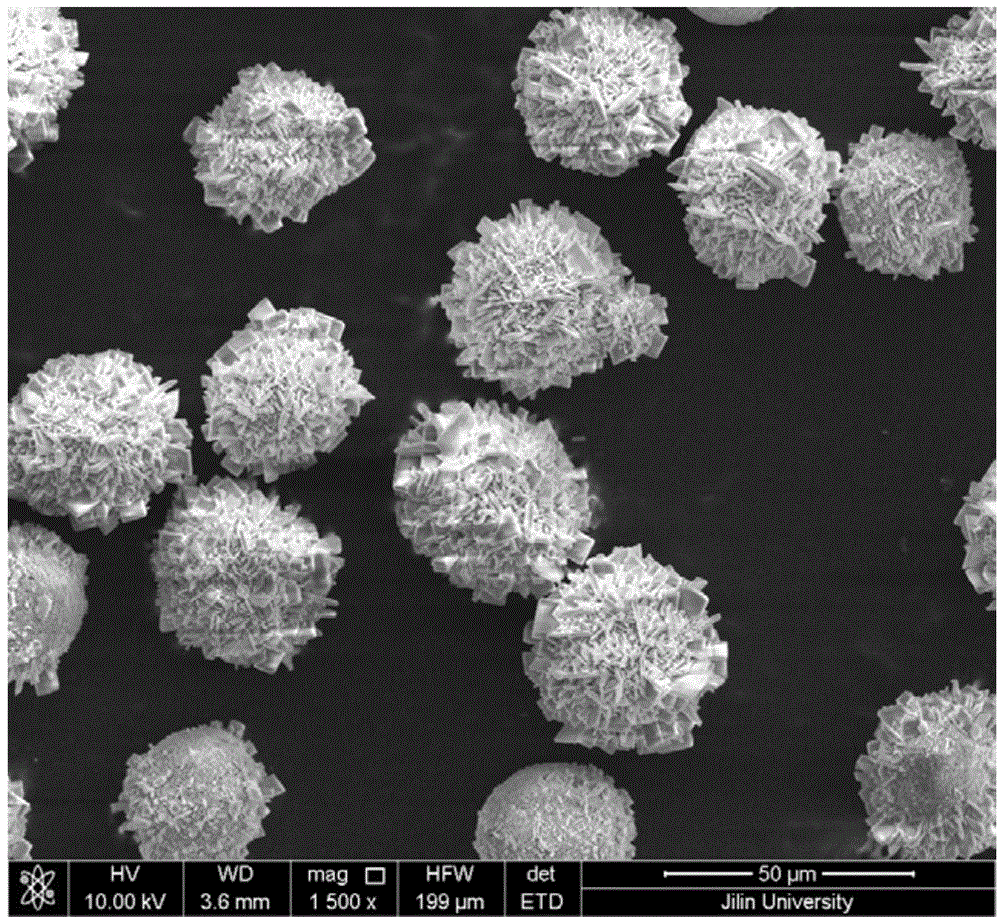
[0045] Weigh 1.14g Na 2 SiO 3 9H 2 O (4mmol) and 10mL distilled water were mixed to make solution A, and dilute hydrochloric acid was added to adjust the pH value to 7, and 0.212g Sr(NO 3 ) 2 (1mmol) and 10mL distilled water were mixed to form solution B. 0.17g CuCl 2 2H 2 O (1mmol) was mixed with 10mL distilled water and stirred (60 minutes), then mixed with 10mL 2mol / L NaOH solution to form suspension C, solution A, solution B and suspension C were mixed. The pH of the solution was adjusted to 11 using concentrated ammonia water. Put it into a high-pressure reactor with a volume of 22.4mL to make the filling degree reach 60%, and react at 250°C for 24 hours after sealing. After the reactor was cooled, the solid phase product was collected. Rinse three times with distilled water and dry. The product is analyzed by a scanning electron microscope to be multi-layered microparticles self-assembled by microsheets. XRD ...
Embodiment 3
[0047] Example 3: SrCuSi 4 o 10 Preparation (3)
[0048] Weigh 1.14g Na 2 SiO 3 ·5H 2 O (4mmol) and 10mL distilled water were mixed to form solution A, and dilute hydrochloric acid was added to adjust the pH value to 9, and 0.2666g SrCl was weighed 2 ·6H 2 O (1mmol) and 5mL distilled water were mixed to form solution B. After solution A and solution B were mixed, 0.08 g of CuO (1 mmol) powder was added and stirred thoroughly (30 minutes), and the pH value of the solution was adjusted to 12.5 with concentrated ammonia water. Put it into a high-pressure reactor with a volume of 22.4mL to make the filling degree reach 80%, and react at 250°C for 12 hours after sealing. After the reactor was cooled, the solid phase product was collected. The product is mixed powder. It was found that the product had some unreacted CuO. After adding 0.5 mol / L HCl solution and soaking for 12 hours, the blue solid phase product was collected, rinsed with distilled water three times, and drie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com