Novel oxazolidinone compound, as well as preparation method and application thereof
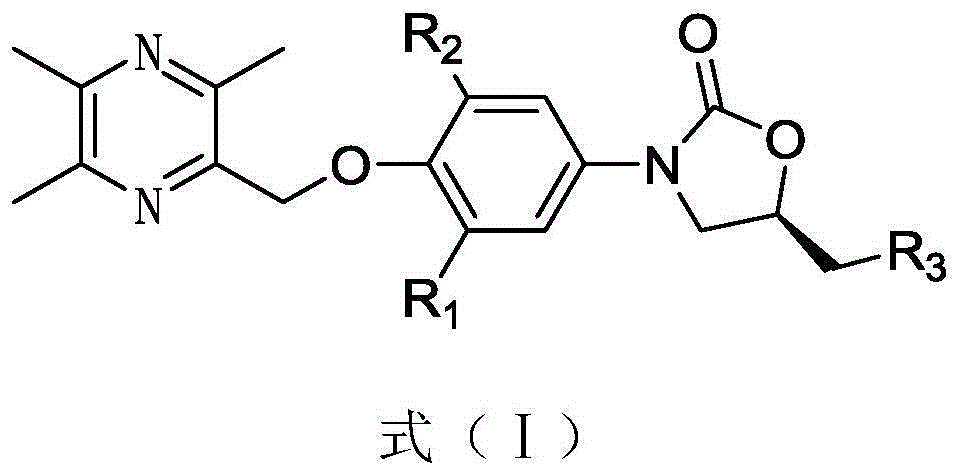
A technology for oxazolidinones and compounds, which is applied in the field of new oxazolidinones and their preparations, and can solve the problems of difficult cross-resistance, weak inhibitory activity of peptidyltransferase, single variety of oxazolidinine antibacterial drugs, etc. problems, to achieve the effect of solving the side effects of hypertensive crisis, improving the activity of dilating blood vessels, and having atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
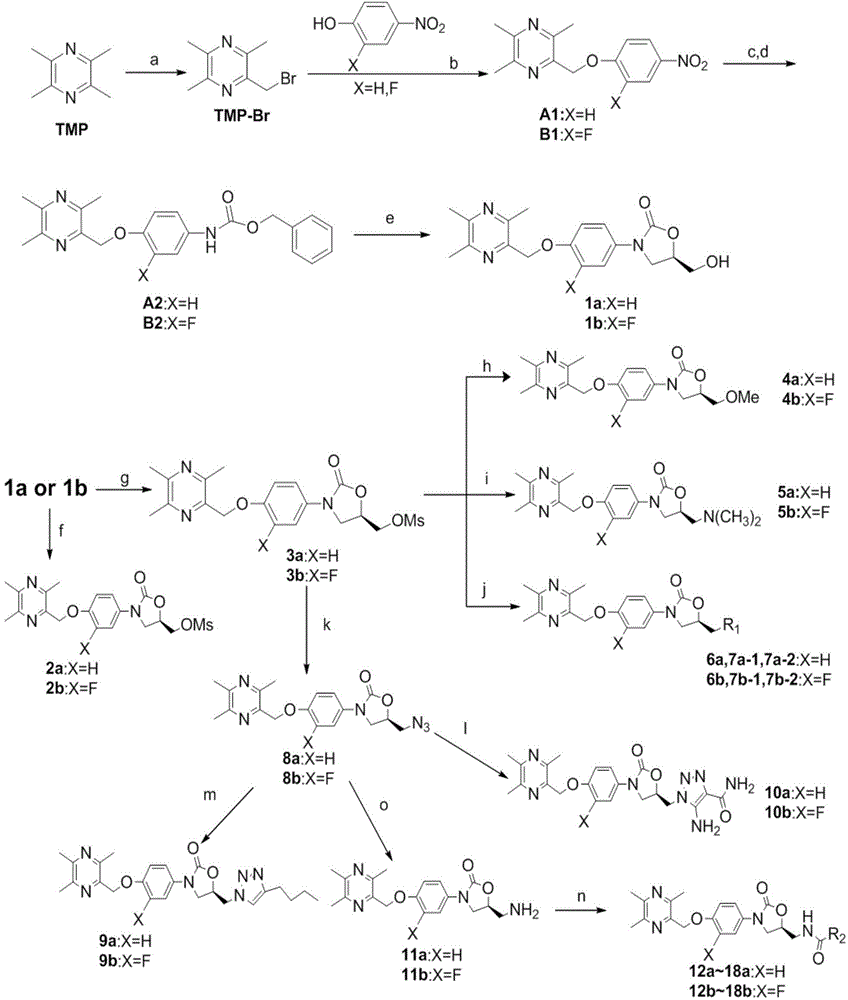
[0046] Example 1: Preparation of 2,3,5-trimethyl-6-((4-nitrophenoxy)methyl)pyrazine (A1)
[0047]
[0048] In the 50mL two-necked flask, add TMP-Br (1.188g, 5.52mmol, TMP-Br is made by known method, can refer to literature Journal of Fudan University, 1980,4:390-394.), p-nitrophenol ( 0.640g, 4.60mmol), potassium carbonate (1.279g, 9.20mmol), and add 5.5mL of anhydrous DMF to dissolve, heat to 85°C, stir for 5-7h, and stop the reaction. The reaction solution was cooled to room temperature, poured into 50 times the volume of ice water, and white flocs were precipitated, filtered to obtain a white solid, and dried in vacuo to obtain a yellowish (log color) solid A1 (1.173 g, 93.4%).
[0049] 1 H NMR (300MHz, CDCl 3 ):δ8.15(d,J=8.0Hz,2H),7.06(d,J=9.1Hz,2H),5.23(s,2H),2.56(s,3H),2.49(s,6H).
Embodiment 2
[0050] Example 2: Preparation of 2-((2-fluoro-4-nitrophenoxy)methyl)-3,5,6-trimethylpyrazine (B1)
[0051]
[0052] Using 2-fluoro-4-nitrophenol and TMP-Br as raw materials, the preparation method was the same as in Example 1 to obtain light yellow solid B2 (6.0 g, 95.2%).
[0053] 1 H NMR (300MHz, CDCl 3 ):δ8.01(ddd, J=9.0,2.6,1.4Hz,1H),7.95(dd,J=10.5,2.7Hz,1H),7.27(dd,J=11.3,5.8Hz,1H),5.33( s,2H),2.59(s,3H),2.50(d,J=2.2Hz,6H).
Embodiment 3
[0054] Example 3: Preparation of benzyl 4-((3,5,6-trimethylpyrazin-2-yl)methoxy)carbamate (A2)
[0055]
[0056] Add A1 (6.0g, 22mmol, the preparation method is the same as in Example 1) and 10%Pd / C (0.6g) into a 100mL two-necked round-bottomed flask, then add THF (35mL) and stir to dissolve. The reaction system was evacuated at 0° C. under a hydrogen atmosphere, replaced with nitrogen twice and once with hydrogen, and then stirred and reacted at room temperature for 24 hours. Filter through diatomaceous earth, wash with acetone 3 times, spin the filtrate to dryness under reduced pressure, and put it directly into the next step. Place the obtained product in the previous step into a 100 mL two-neck round bottom flask, and use 4 mL of H 2 O dissolved sodium carbonate (2.6g, 2.2mmol) into the reaction flask, then added 40mL of acetone, and slowly added benzyloxycarbonyl chloride (10mL, 66mmol) dropwise under a nitrogen atmosphere. After reacting at room temperature for 18 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com