Preparation method of N-(phosphonomethyl) iminodiacetic acid
A technology of bisphosphonate and monosodium iminodiacetate, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc. Reaction and other problems, to achieve the effect of reducing sodium chloride, reducing sodium chloride content and improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
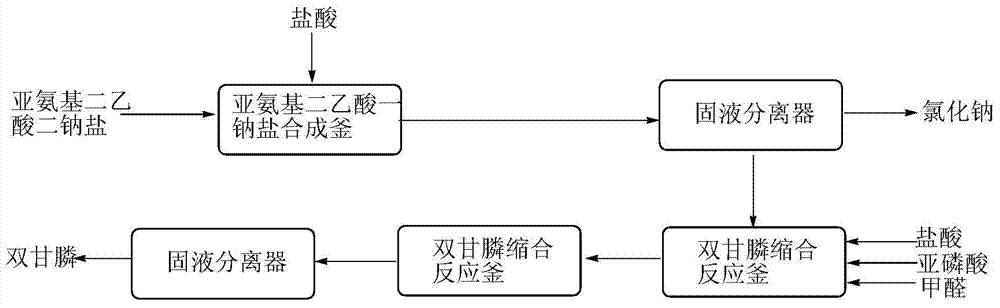
[0029] The embodiment of the present invention discloses a preparation method of bisglyphosate, comprising the following steps:
[0030] (A) react disodium iminodiacetic acid with hydrochloric acid to obtain a solution containing monosodium iminodiacetic acid and sodium chloride;
[0031] (B) Concentrating the solution containing monosodium iminodiacetic acid and sodium chloride, then heating and filtering to obtain sodium chloride solid and filtrate;
[0032] (C) Mix the filtrate with hydrochloric acid and phosphorous acid, heat, then add formaldehyde, and react to obtain bisglyphosate.
[0033] The invention uses disodium iminodiacetate as a starting material to prepare diglyphosate. The source of the disodium iminodiacetate is not particularly limited, and it can be obtained commercially or preferably obtained by hydrolysis of iminodiacetonitrile or oxidation of diethanolamine. The method for obtaining disodium iminodiacetonitrile by hydrolyzing iminodiacetonitrile is as ...
Embodiment 1
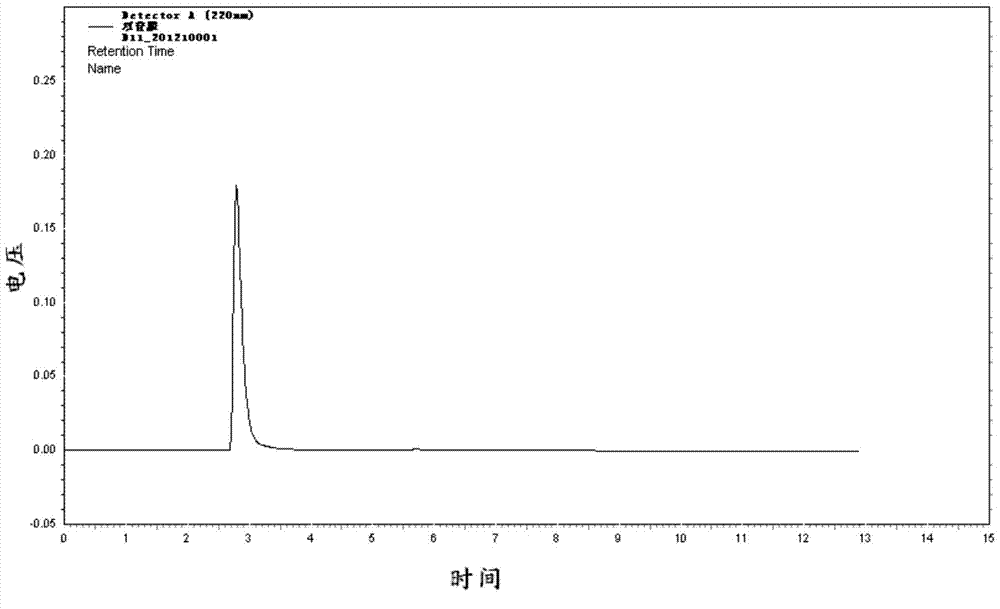
[0043] Put 179.6g (0.45mol) of 44.5% iminodiacetic acid disodium salt produced after dehydrogenation of diethanolamine into a four-neck flask, heat to 70°C to dissolve, and add 31% hydrochloric acid dropwise under stirring to neutralize to the pH value 5.0, concentrated under reduced pressure to distill 90g of water, raised the temperature to 80°C and filtered while hot, washed the filter cake with 20mL of hot water, dried to obtain 14.3g of solid, of which the mass content of iminodiacetic acid monosodium salt was 0.10%, and the rest was chlorinated sodium.
[0044] The filtrate was transferred to a four-necked bottle equipped with a reflux condenser, a thermometer, an agitator, and a constant pressure dropping funnel, and 45.7 g of phosphorous acid (97% by mass, 0.54mol), 111.9 g of hydrochloric acid (97% by mass) were added. 31%, 0.95mol), temperature control between 115-120℃, add 47.0g formaldehyde (content 37%, 0.58mol) dropwise, keep warm for 2h after adding, cool down t...
Embodiment 2
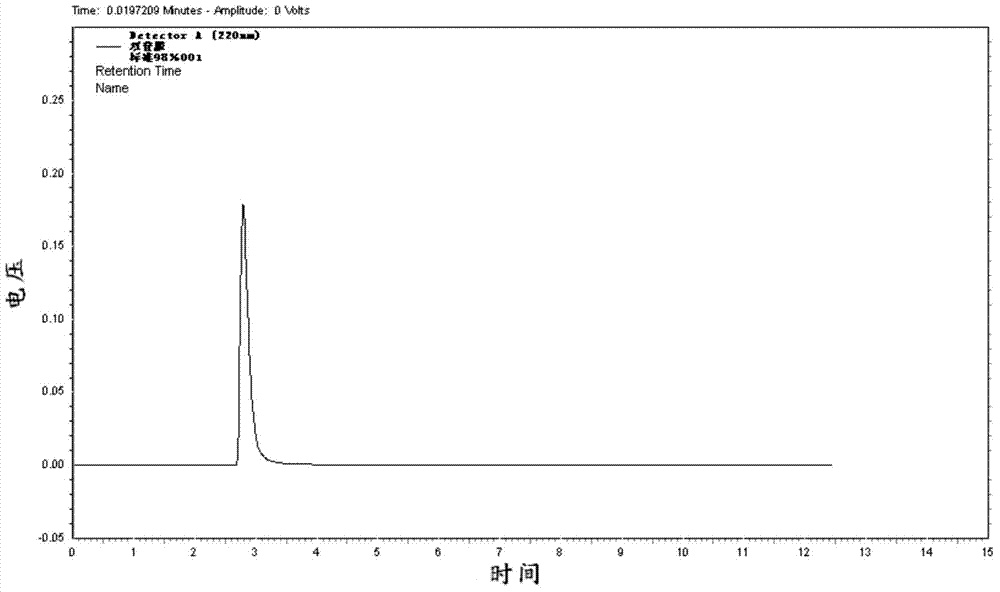
[0048]Put 426.5 g (content 41.5%, 1 mol) of iminodiacetonitrile disodium salt generated after hydrolysis of iminodiacetonitrile sodium hydroxide solution into a four-neck flask, heat to dissolve, and add 36% hydrochloric acid dropwise under stirring conditions to neutralize to The pH value is 5.2, concentrated under reduced pressure to distill 250g of water, heated to 85°C and filtered while hot, the filter cake was washed with 20mL of hot water, and dried to obtain 19.8g of solid, of which the mass content of iminodiacetic acid monosodium salt was 0.11%, and the rest was Sodium chloride.
[0049] The filtrate was transferred to a four-necked bottle equipped with a reflux condenser, a thermometer, an agitator, and a constant pressure dropping funnel, and 97.2 g of phosphorous acid (97% by mass, 1.15mol), 294.5 g of hydrochloric acid (31% by mass) were added. , 2.15mol), add 105.4g formaldehyde dropwise between 115-120°C (content 37%, 1.3mol) and keep warm for 2h, cool down to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com