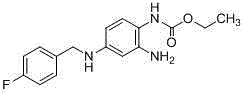
An intermediate of n1-(4-fluorobenzyl)-4-nitrophenyl-1,3-diamine and its preparation method
A technology of nitrophenyl and fluorobenzyl, applied in the field of N1--4-nitrophenyl-1,3-diamine intermediate and its preparation, can solve the problem of long reaction time and high boiling point of DMSO, which is difficult to remove , low yield and other problems, to achieve the effects of short reaction time, high reaction selectivity and yield, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
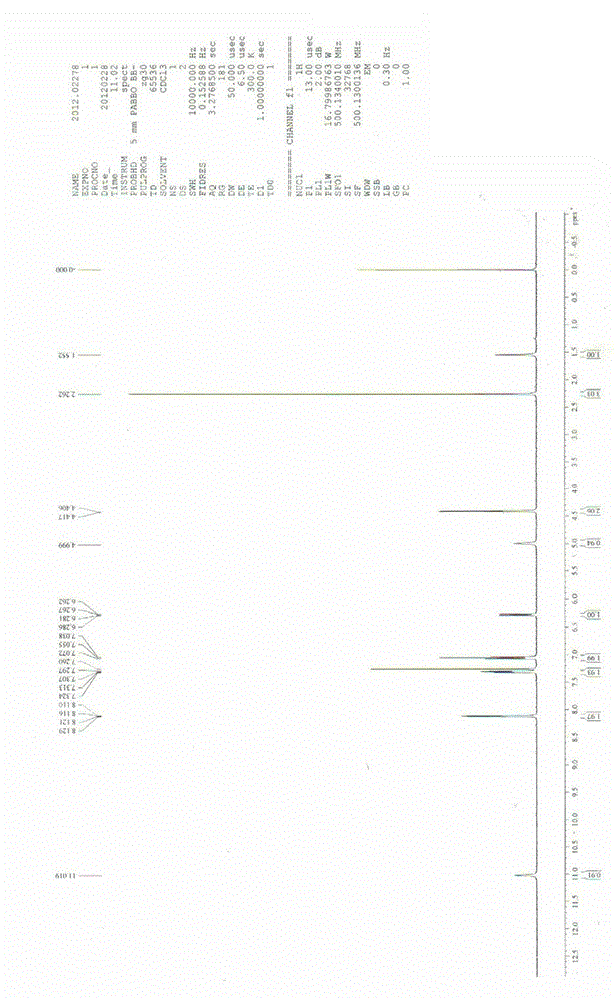
[0068] The chemical structural formula of compound (VI): .
[0069] The melting point of compound (Ⅵ) is 170 ~ 173 ℃; the hydrogen spectrum features are: 1 H NMR (500MHz, CDCl 3 ) δ: 11.0 [s , 1H , Ar- NH -C(=O)CH 3 ] , 8.12 (dd , J = 6.5 , 4.0 Hz , 2H , ArH) , 7.30 (dd , J = 8.5, 5.0 Hz , 2H , ArH) , 7.05 (t , J = 8.5 Hz , 2H , ArH) , 6.27 (dd , J = 9.5, 2.5 Hz, 1H, ArH) , 5.00 (s , 1H , Ar- CH 2 -NH-Ar) , 4.41 (d , J = 5.5 Hz , Ar-CH 2 - NH -Ar) , 2.26 [s , 3H, -(C=O)CH 3 ]. NMR detection features: ESI-MS m / z : 304 [M+H] + , as attached figure 1 shown.
Embodiment 2
[0071] Synthesized by compound (Ⅵ) N 1 -(4-fluorobenzyl)-4-nitrophenyl-1,3-diamine method.
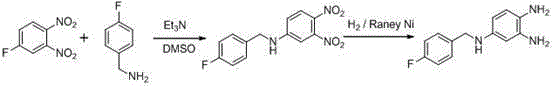
[0072] Its specific reaction is:
[0073]
[0074] Step 1: nitrification reaction: the N -(3-fluorophenyl)acetamide (compound Ⅲ, Hal=F) (76.5 g, 0.5 mol), ethylene glycol dimethyl ether (200 mL), concentrated nitric acid (65 mL, 1.1 mol) were added to 1L The reaction bottle was stirred, and concentrated sulfuric acid (200 mL, 3.6 mol) was added dropwise at room temperature. After 1.5 hours, the reaction solution was added dropwise into crushed ice, and a white solid precipitated out. Then add 1L of water, stir for 15 minutes and filter, wash the filter cake with water to obtain 88g of white solid, recrystallize the resulting crude product with petroleum ether: ethyl acetate = 1.1: 1, filter with suction, concentrate the mother liquor to obtain a white solid N -(5-fluoro-2-nitrophenyl)acetamide (compound IV, Hal=F) 74.2 g, yield 75%.
[0075] Compound (Ⅳ): mp 86 ~ 87 ℃. 1 H NMR...
Embodiment 3~7
[0082] Embodiment 3~7 to the selection of step-nitration reaction condition
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com