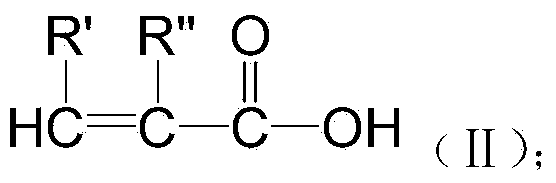
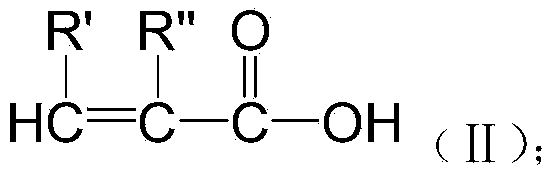
Preparation method of biomedical polymer material of polyunsaturated acid gallic acid epoxy ester
A polymer material, acid epoxy ester technology, applied in the direction of non-polymer adhesive additives, grafted polymer adhesives, adhesive types, etc. The effect of high oxygen levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Epoxy Ester of Unsaturated Acid Gallate:
[0036]Take 1 mole of gallic acid and put it into a reaction bottle, add 20 moles of epichlorohydrin and 1 mole of n-butanol as solvents and stir and mix, then gradually heat up and add 4 moles of 20% sodium hydroxide aqueous solution dropwise, at 90 degrees Celsius React for 6 hours, wash with deionized water to neutrality, and distill off water and unreacted epichlorohydrin under reduced pressure to obtain gallic acid epoxy resin; and its epoxy value is measured to be 0.8mol / 100g.
[0037] Take 50 grams of the above-mentioned gallic acid epoxy resin and add it to the reaction bottle, then add 1.5 times the molar amount of α-methacrylic acid according to the molar amount of epoxy groups, and then add 0.2 grams of N,N' dimethyl benzyl Amine and 0.01 gram of hydroquinone were slowly heated to 90 degrees centigrade and reacted for 8 hours under stirring, cooled to room temperature, washed with deionized water to rem...
Embodiment 2
[0039] Preparation of Epoxy Ester of Unsaturated Acid Gallate:
[0040] Get 1 mole of gallic acid and put it into a reaction bottle, add 40 moles of epichlorohydrin and 2 moles of isoamyl alcohol and stir and mix, then gradually heat up and add dropwise 4 moles of 20% sodium hydroxide aqueous solution, and react at 85 degrees Celsius for 6 hours, washed with deionized water to neutral, and evaporated water and unreacted epichlorohydrin under reduced pressure to obtain gallic acid epoxy resin; and its epoxy value was measured to be 0.9mol / 100g.
[0041] Add 50 grams of gallic acid epoxy resin to the reaction bottle, then add 1.5 times the molar amount of acrylic acid according to the molar amount of epoxy groups, then add 0.1 gram of N,N' dimethylaniline and 0.05 gram of hydroquinone, and slowly Raise the temperature to 100 degrees Celsius and react for 6 hours, cool to room temperature, wash off unreacted carboxylic acid with deionized water, and vacuum dehydrate to obtain uns...
Embodiment 3
[0043] Add 10 grams of polymethyl methacrylate powder in the 50 g α-gallic acid epoxy ester synthesized in Example 1, add 2 grams of ultraviolet photoinitiator TPO and 1.5 grams of silicon coupling agent-processed micron dioxide Silicon, 1 gram of 4-[β-3,5-di-tert-butyl-4-hydroxyphenyl) propionate] pentaerythritol ester, mixed evenly and put into the mold, irradiated with 800W UV light source for 30 minutes to cure. The glass transition temperature of the prepared material was determined to be 100 degrees Celsius, and the Rockwell hardness was 60.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
| Epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com