Esterase, gene, vector and engineering bacterium derived from thermomyces lanuginosus as well as application
A technology of Thermomyces sp. and genetically engineered bacteria, applied in the field of esterase, can solve the problem of high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Acquisition of T.lanuginosus DSM10635 esterase gene
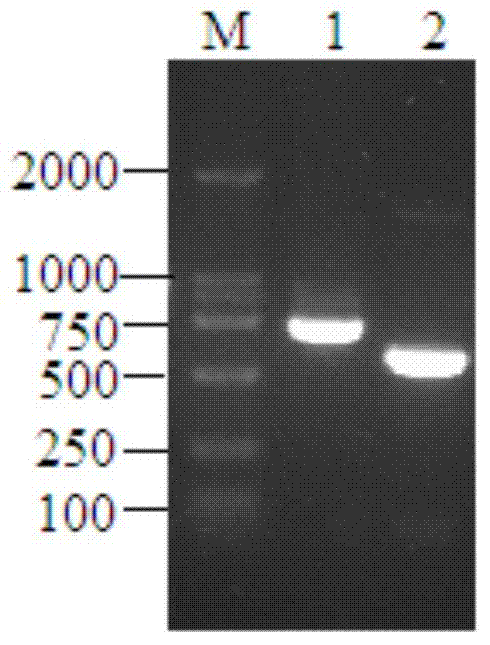
[0052] Collect the mycelia of T.lanuginosus DSM10635 (from the German Collection of Microorganisms and Cell Cultures (DSMZ)) cultured at 50°C for 3 days, add liquid nitrogen to fully grind to powder, extract total RNA by TRIzol method, and use Promega’s GoScript TM The reverse transcription kit is used to prepare the first strand of cDNA, and the reaction system and conditions refer to the instructions of the kit. Then use primer 1 (GGSTTYASYKCNGGNGG) and oligodT designed according to a conserved sequence of fungal esterase as primers, and use cDNA as a template to carry out PCR amplification under the action of LA-Taq DNA polymerase. The amount of each component in the PCR reaction system (total volume 50 μL): 10×LA-Taq DNA Polymerase Buffer 5 μL, 10 mM dNTP mixture (dATP, dCTP, dGTP and dTTP each 2.5 mM) 0.5 μL, primer 1 and primer oligodT at a concentration of 50 μM 0.5 μL each, 1 μL cDNA, 0.5 μL LA-Ta...
Embodiment 2
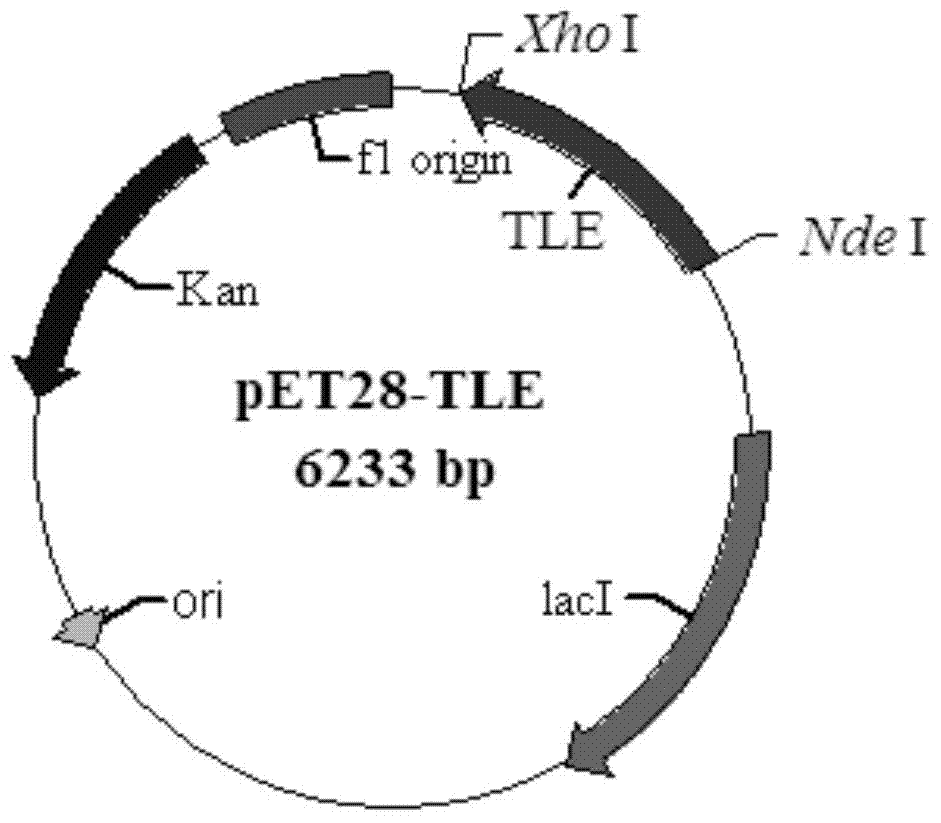
[0053] Example 2: Construction of recombinant expression vector pET28-TLE
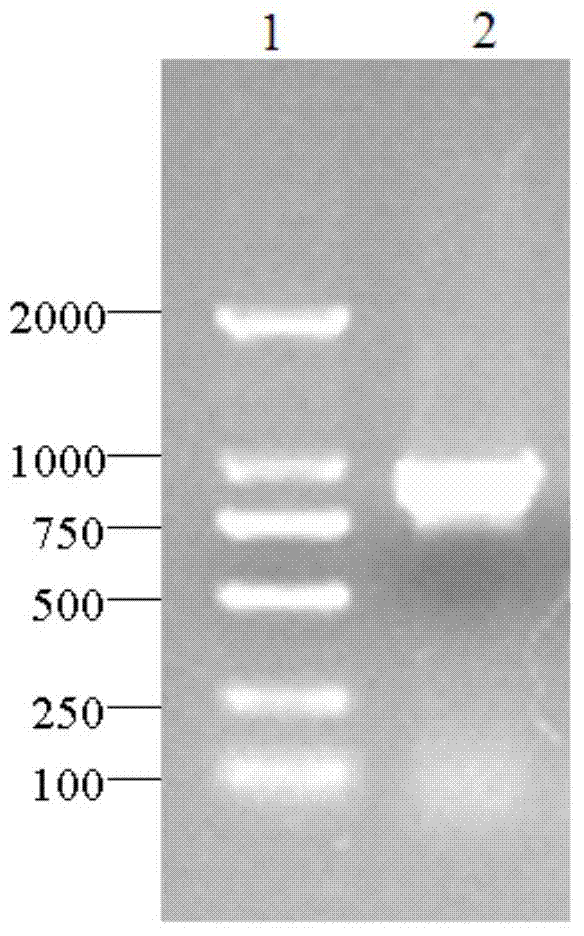
[0054] According to the analysis result of embodiment 1, design expression primer 4 (GGAATTC CATATG GGCTTGTTTTCAATCCTG), Primer 5 (CCG CTCGAG TTATCCACTGTGGCCAAACTC). Initiated by primer 4 and primer 5, use high-fidelity Pfu DNA polymerase (TaKaRa) to amplify, the amount of each component of the PCR reaction system (total volume 100 μL): 10×Pfu DNA Polymerase Buffer 10 μL, 10 mM dNTP mixture (dATP , dCTP, dGTP and dTTP each 2.5 mM) 0.5 μL, 0.5 μL each of primer 4 and primer 5 at a concentration of 50 μM, 1 μL of cDNA, 1 μL of Pfu DNA Polymerase, and 86.5 μL of nucleic acid-free water. The PCR instrument of Biorad was used, and the PCR reaction conditions were: pre-denaturation at 94°C for 3 minutes, then entering into a temperature cycle of 94°C for 30s, 60°C for 30s, and 72°C for 10 minutes, a total of 30 cycles, and finally extending at 72°C for 10 minutes, and the termination temperature was 8°C....
Embodiment 3
[0056] Embodiment 3: Construction of engineering bacteria E.coli BL21(DE3) / pET28-TLE
[0057] The recombinant expression vector pET28-TLE constructed in Example 2 was transformed into Escherichia coli BL21(DE3), spread on an LB plate containing 50 μg / ml Kan and cultured at 37°C overnight, randomly picked clones for colony PCR identification, positive clones Sequencing verification showed that the recombinant expression vector pET28-TLE was successfully transformed into the expression host E.coli BL21(DE3), and the esterase gene was successfully cloned into the NdeI and XhoI sites of pET-28b.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com