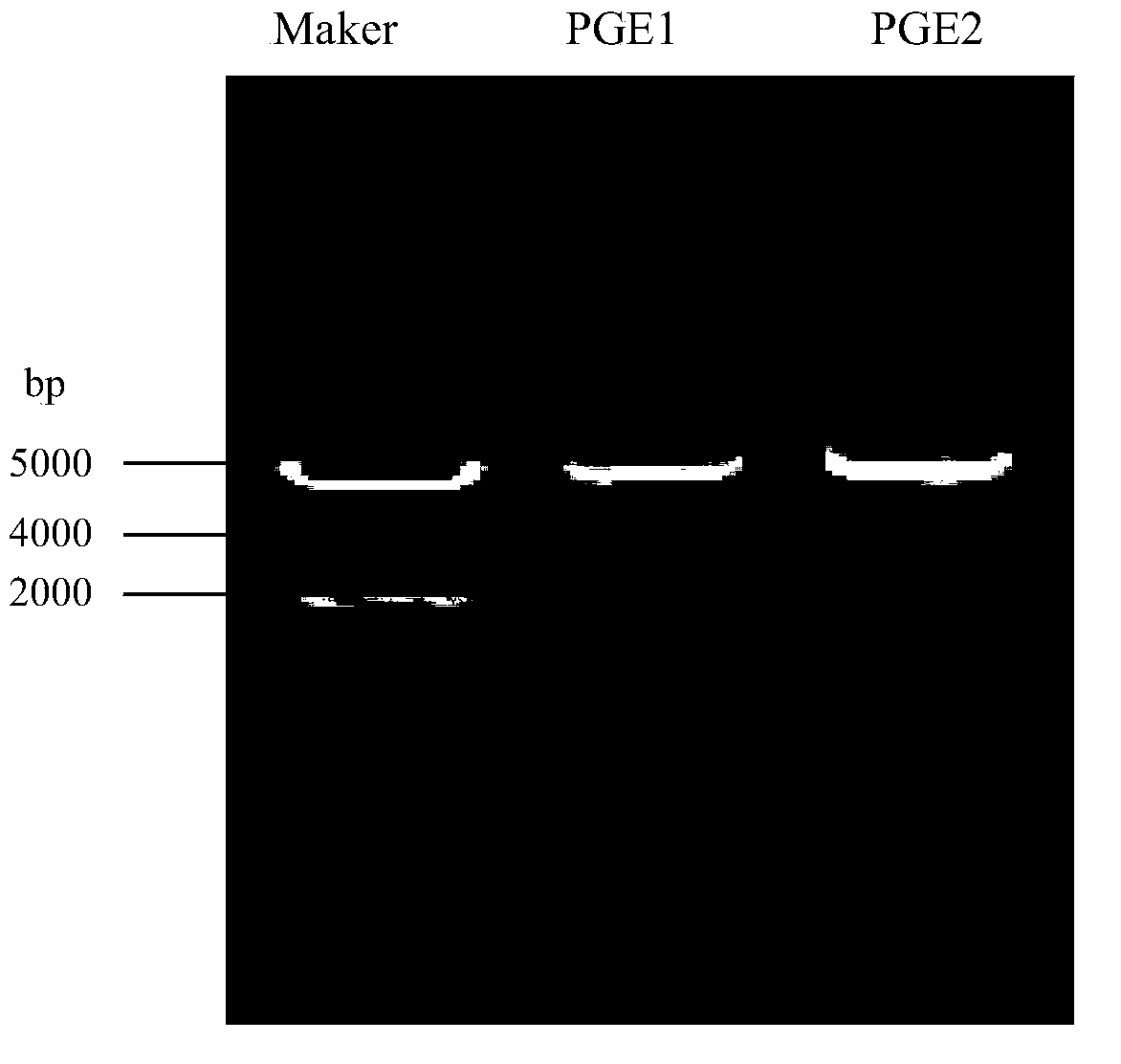
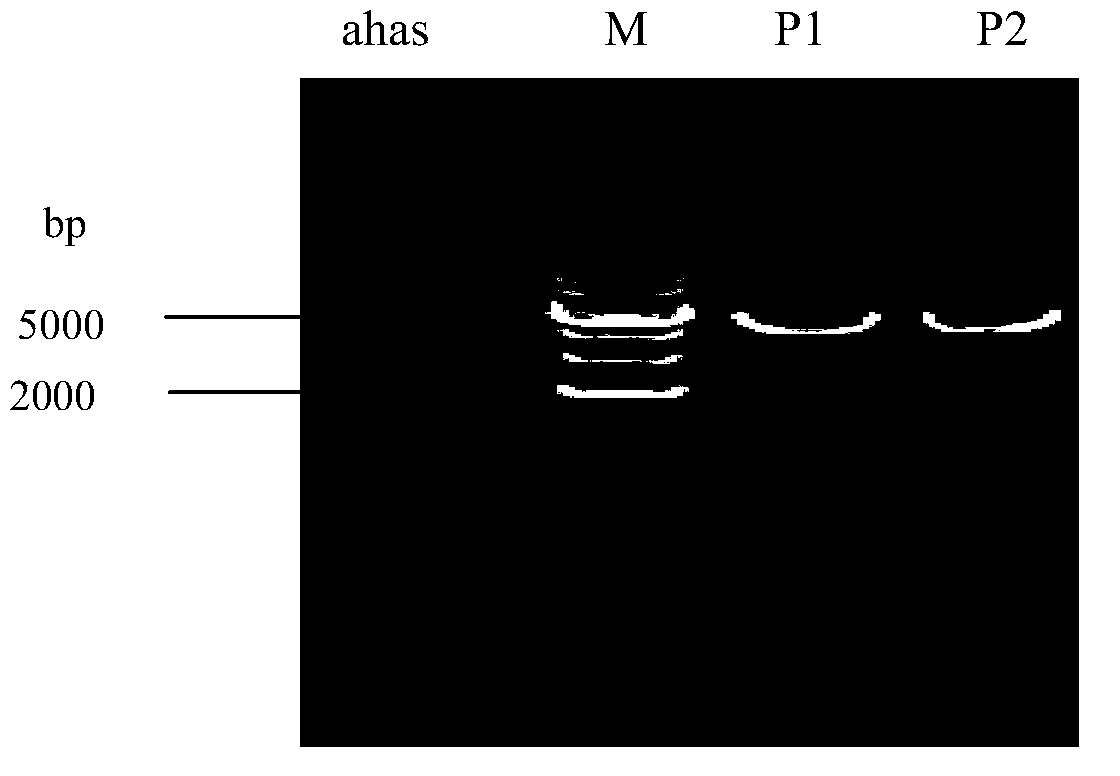
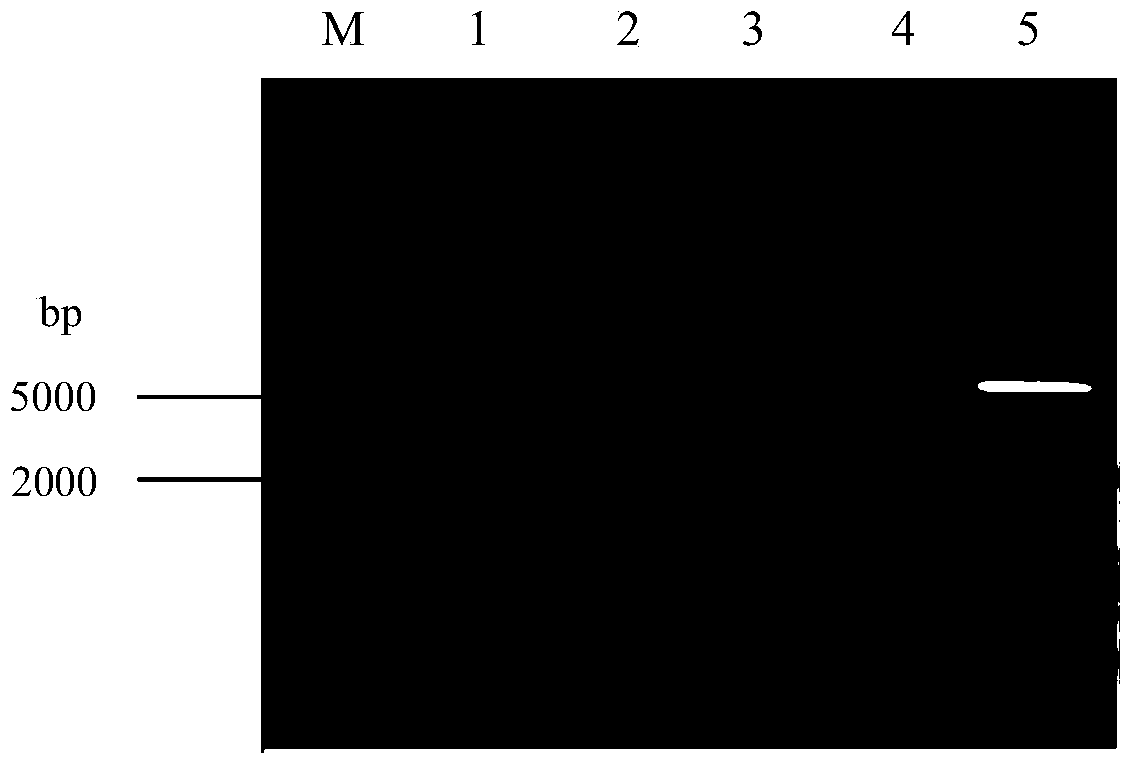
Preparation and preservation method for colon bacillus acetohydroxyacid synthase
A technology of acetolactate synthase and Escherichia coli, applied in the field of genetic engineering, can solve the problems of poor enzyme stability, complicated operation and low AHAS protein content, and achieve the effects of good stability, high purity and good activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0056] Main materials used in this embodiment:
[0057]
[0058]
[0059] Preparation of media and reagents:
[0060] (1) LB (Luria-Bertani) liquid medium: Weigh 5g yeast extract, 10g tryptone, 10g NaCl, dissolve in 800mL double distilled water (ddH 2 O), then adjust the pH to 7.0 with 2M NaOH, and then use ddH 2 O diluted to 1L, autoclaved at 121°C for 20min, and stored at 4°C for use; (2) LB solid medium: weigh 0.5g yeast extract, 1g tryptone, 1g NaCl, 1.5g agar, and dissolve in 90mL wxya 2 O, then adjust the pH to 7.0 with 2M NaOH, and then use ddH 2 Dilute O to 100mL, autoclave at 121°C for 20min, wait for the liquid to cool to about 60°C, pour it into 6 sterilized petri dishes on average, and store it at 4°C after cooling for later use;
[0061] (3) Ampicillin (Amp) solution: weigh 0.1g of Amp, dissolve it in 1mL of sterile water to make the final concentration 100mg / mL, and store it at -20°C after aliquoting;
[0062] (4) Isopropyl-β-D-thiogalactopyranoside (I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com