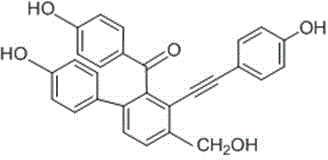
Compound with antifungal activity and preparation method and application thereof
An antifungal drug and antifungal technology, applied in the directions of antifungal agents, separation/purification of carbonyl compounds, organic chemistry, etc., can solve the problems of human toxicity and side effects, treatment failure, etc., and achieve the effect of convenient preparation and convenient clinical use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of compound II with antifungal activity provided by the invention, it may further comprise the steps:
[0026] (1) Take the dried Selaginella matta, crush it, add 10 times the concentration of 95% ethanol to reflux and extract 3 times, each time for 2 hours, combine the extracts, concentrate under reduced pressure until there is no alcohol smell, dilute with water, then use petroleum ether, Extraction with ethyl acetate and water-saturated n-butanol, take the ethyl acetate extract and recover the solvent under reduced pressure to obtain the total ethyl acetate extract;
[0027] (2) Take the ethyl acetate extract (108g) prepared in step (1), put it on silica gel column chromatography, use chloroform:methanol whose volume ratio is 95:5~50:50, gradient elution successively, thin-layer chromatography detection Identify, combine the fractions of the same spot; get six fractions (Fr.1~Fr.6), put Fr.2 on silica gel column chromatography, use the volume r...
Embodiment 2
[0032] Embodiment 2 antifungal activity experiment
[0033] 1. The present invention conducts in vitro antifungal tests on compound II, which shows that it has a more obvious effect of inhibiting fungi. The cell strains used in the test are ATCC standard strains provided by the Fungal Center of the Medical Microbial Bacteria (Virus) Species Preservation Management Center of the Ministry of Health, including:
[0034] Candida genus: Candida albicans (Candida albicans) bacteria number: CMCCC(F)C .1L (ATCC90028)
[0035] Aspergillus: Aspergillus fumigatus (Aspergillus fumigatus) No.: CMCCC(F)A 1g (ATCC-MYA-3626)
[0036] Trichophyton genus: Trichophyton rubrum No.: CMCCC(F)T .1h (ATCC-MYA-4438)
[0037] Trichophyton mentagrophytes bacteria number: CMCCC(F)T .5e (ATCC-MYA-4439)
[0038] A total of 3 genera, 4 species and 4 strains of common pathogenic fungi were identified.
[0039] 2. Specific experimental methods The experiments were conducted with reference to the standar...
Embodiment 3 Embodiment 1
[0048] Example 3 Acute Toxicity Test of Compound II Gained in Example 1
[0049] Calculate the half lethal dose LD of mice according to Bliss method 50 value, LD 50 The value is 1.42 mg / kg, and the experimental results show that the compound II provided by the present invention has low acute toxicity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com