Fullereneporphyrin derivate photosensitizer as well as preparation method and application thereof
A technology of fullerene porphyrins and derivatives, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., which can solve the problem of affecting the efficiency of active oxygen, reducing the effect of photodynamic therapy, and easy aggregation of photobleaching and other problems, to achieve the effect of increasing the probability of interaction, significant killing effect, and high phototoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
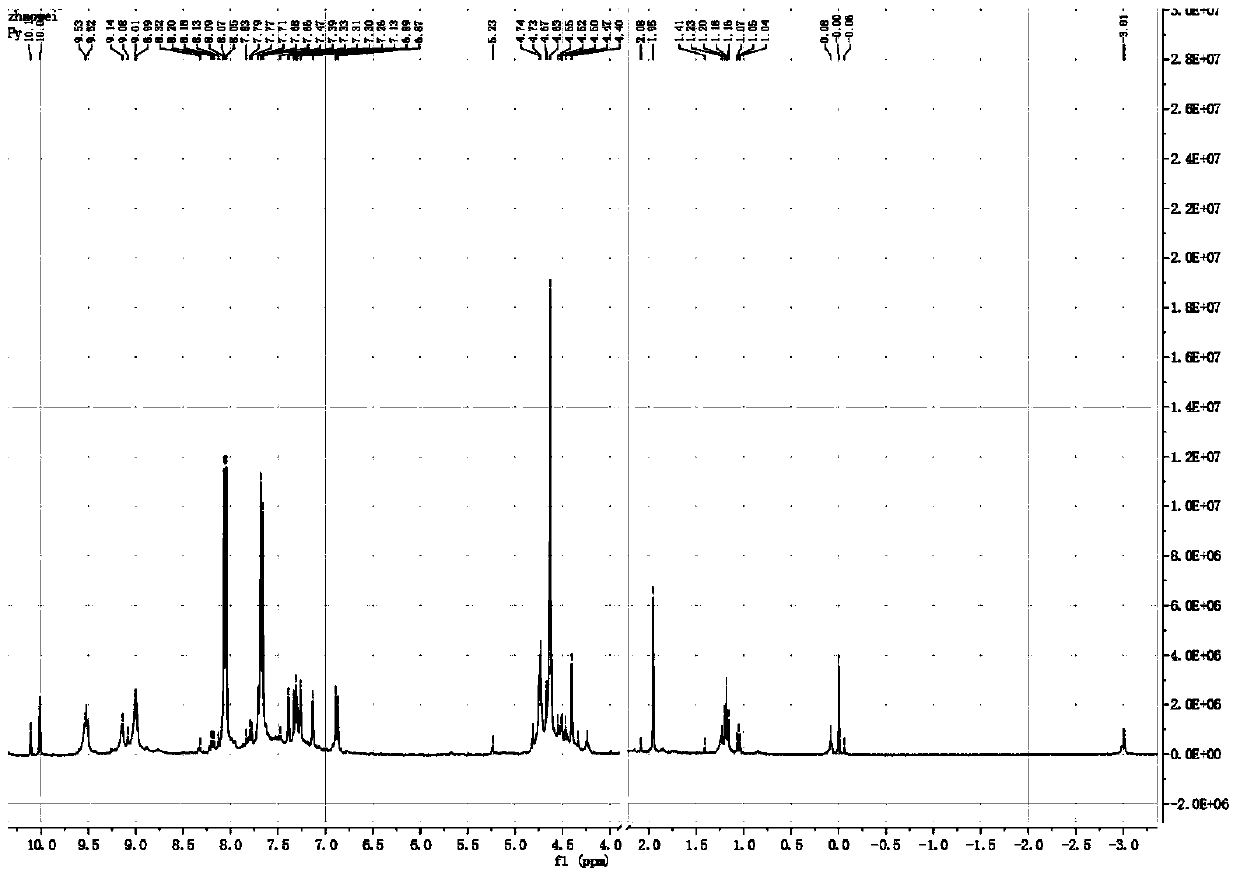
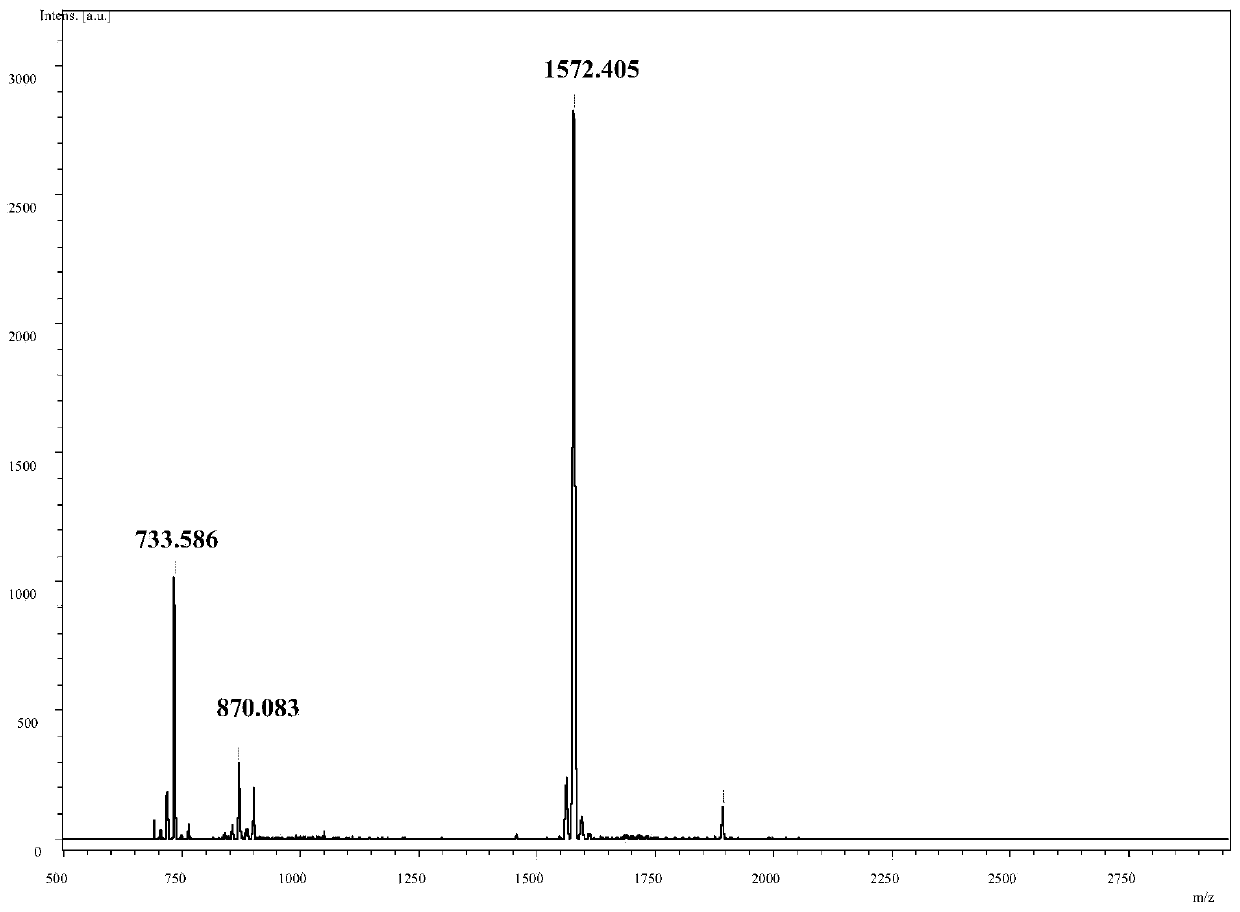
[0040] Preparation of fullerene porphyrin derivatives shown in embodiment 1, formula I
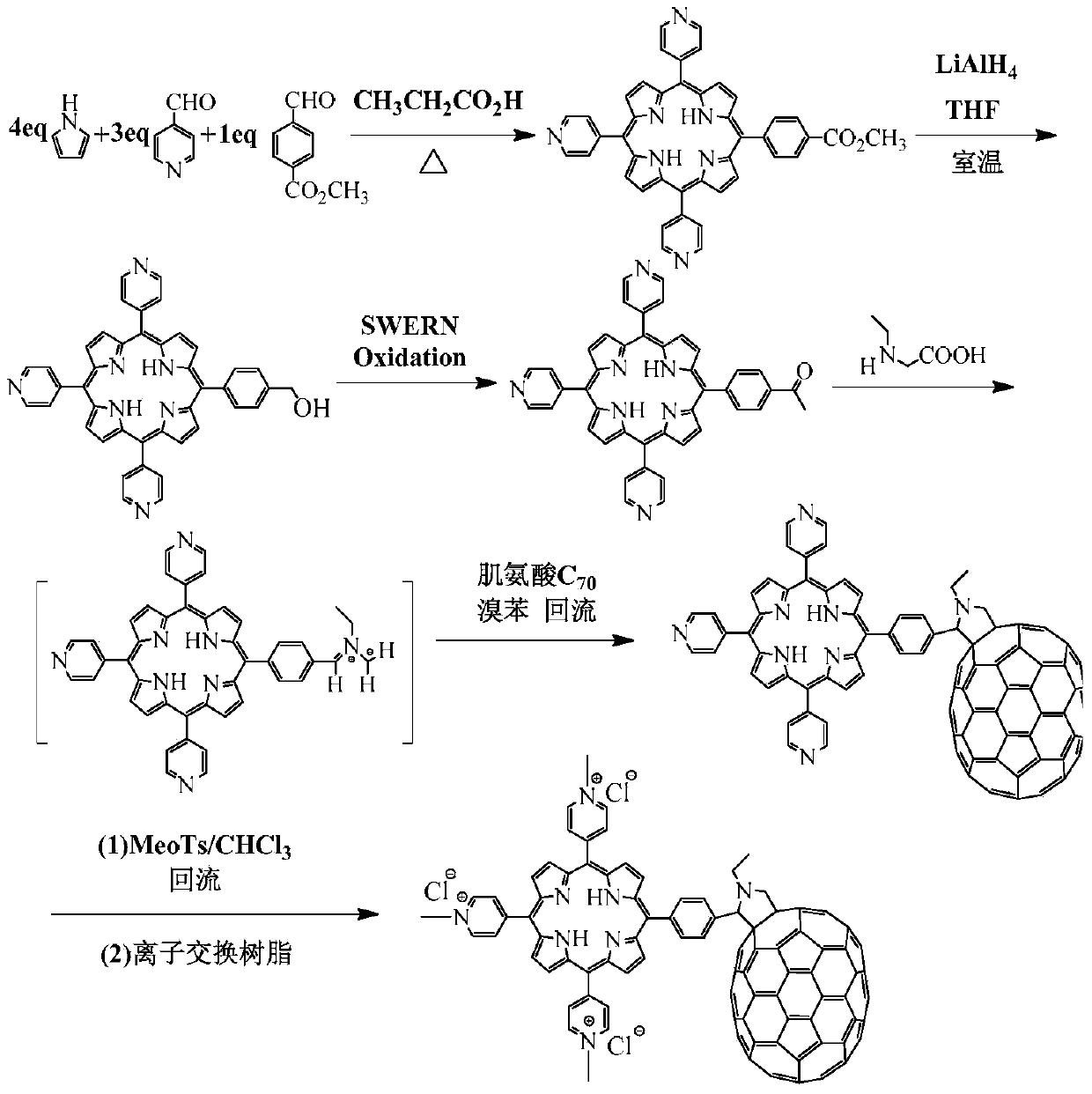
[0041] The reaction equation is as figure 1 shown.
[0042] (1) Methyl p-formylbenzoate, 4-pyridinecarbaldehyde and pyrrole are mixed according to 1:3:4 (molar ratio), using propionic acid as a solvent, heated to reflux for 1.5h, and the reacted mixture is first recrystallized with methanol , purified by silica gel column separation (eluent: trichloromethane:methanol=98:2, v / v) to obtain the compound shown in formula 1.
[0043] (2) The compound shown in formula 1 and LiAlH4 The molar ratio of substances was 1:8, reacted at room temperature (25°C), and the reactant was monitored by column chromatography (developing solvent: chloroform:ethanol=97:3, v / v) to obtain the compound shown in formula 2.
[0044] (3) The compound shown in formula 2 oxidized the hydroxyl group to an aldehyde group through the Swern oxidation reaction, and the specific steps were as follows: 0.5 mL of oxalyl chlori...
Embodiment 2
[0048] Example 2, Evaluation of the Toxic Effect of Fullerene Porphyrin Derivatives Shown in Formula I on A549 Cells
[0049] (1) Resuscitate A549 cells (Cell Resource Center, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences), adjust the cell density to 5×10 4 / mL, seed A549 cells into 96-well plates (6×6), at 37°C, 5% CO 2 Incubate for 24h under the condition;
[0050] (2) After the cells adhere to the wall, replace with DMEM (Corning R10-013-CV, purchasing company: Baierdi) solution containing different concentration gradients of fullerene porphyrin derivatives shown in formula I at 37°C, 5% CO 2 The culture was continued for 24 h under the same conditions.
[0051] According to the instructions for use, use cck-8 to detect the activity of A549 cells, the dark toxicity test results of fullerene porphyrin derivatives shown in formula I to A549 cells are as follows Image 6 (B) shown.
[0052] Depend on Image 6 (B) It can be known that fullerene ...
Embodiment 3
[0053] Example 3, Photodynamic Killing Effect of Fullerene Porphyrin Derivatives Shown in Formula I on A549 Cells
[0054] (1) Resuscitate A549 cells and adjust the cell density to 5×10 4 / mL, seed A549 cells into 96-well plates (6×6), at 37°C, 5% CO 2 Incubate for 24h under the condition;
[0055] (2) After the cells adhered to the wall, they were replaced with DMEM solutions containing different concentration gradients of fullerene porphyrin derivatives shown in formula Ⅰ at 37°C and 5% CO 2 Continue culturing for 3 h under the condition;
[0056] (3) Remove the medium containing the fullerene porphyrin derivative shown in formula I, and replace it with fresh colorless DMEM at 20mW / cm -2 Under the light intensity of 10min, replace the fresh medium and continue to incubate for 24h.
[0057] According to the instructions for use, use cck-8 to detect the activity of A549 cells, the phototoxicity test results of fullerene porphyrin derivatives shown in formula I to A549 cell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com